MOJ
eISSN: 2373-4442


Research Article Volume 3 Issue 3
1Botany Department, Ain Shams University, Egypt
2Microbiology and Immunology Department, Al-Azhar University, Egypt
3Late Director of the Regional Center for Mycology & Biotechnology, Al-Azhar University, Egypt
Correspondence: Moustafa Abdelnasser,Microbiology and Immunology Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt, Tel +201116189541
Received: May 01, 2016 | Published: May 13, 2016
Citation: Mohamed GM, Kheiralla ZM, Nasser MA, AbuSeada AERA (2016) Effect of Dermatophytes on Neutrophils and Monocytes Chemotaxis. MOJImmunol 3(3): 00089. DOI: 10.15406/moji.2016.03.00089
Introduction: Dermatophytes are a specialized group of fungi which affect keratinous tissue of humans and other vertebrates, causing superficial infections.
Objectives: The current study aimed at investigating the chemotactic activity of a group of dermatophyte fungi towards neutrophils and monocytes.
Material and methods:Fifty-three patients with superficial fungal infections of glabrous skin i.e. Tinea cruris, T. capitis, T. corporis and T. pedis were investigated. They were 28 females and 25 males aged 3-66 years old (average 32.7±15.3). In the present work, 26.6% (14/53) of these patients were suffering from T. corporis followed by T.pedis in 22.6% (12/53), T.versicolor in 22.6% (12/53), T.cruris in18.8% (10/53)and T.capitis in 9.4% (5/53) of them. Fungal examination of the scaly lesions showed the presence of the following dermatophytes: Microsporum canis, Trichophyton rubrum, T. verrocosum and T.mentagrophytes. Neutrophils and monocytes separated from apparently healthy donors were tested for their migrating abilities using the under agarose (Nelson) technique. Different concentrations of fungal mycelia with or without normal serum were used in parallel to negative (tissue culture medium) and positive (zymosan activated serum) chemo attractants. Cell migration was measured by the leukotactic index (LI); the ratio of migration towards test/migration towards control.
Results: Most of the tested dermatophyte concentrations were stimulatory for either neutrophil or monocyte chemotaxis (LI>1.0). It seemed that T.verrocosum was the most stimulatory one. Neutrophils were more actively migrating than monocytes as the differences between LIs of these cells were more than LSD at 5% (least significant difference). A non proportional dose-effect relationship between fungal concentration and chemotactic activity was observed.
Conclusion: These data stress on the important role of neutrophil and monocyte in host defense against dermatophyte infection. The data also indicate that neutrophil is a more active responder to fungal infection than monocyte.
Keywords: neutrophils, monocytes chemotaxis, SDA, fungal infections, LI, monocytes, phagocytic cells, superficial infections, cell migration
SDA, sabouraud’s dextrose agar; RCMB, regional center for mycology and biotechnology; ZAS, zymosan activated serum; TC, tissue culture; LI, leukotactic index; SM, spontaneous migration; PBS, phosphate buffered saline
Chemotaxis is a reaction by which the direction of locomotion of cells is determined. It is a directed cellular migration along a concentration gradient of chemo attractant. If the cells are moving towards higher concentration of the attractant, chemotaxis is said to be positive. Chemotaxis in leukocytes is in contrast to bacteria which also exhibit negative chemotaxis.1 Likewise Fungi seems to behave like bacteria. PMNs and macrophages are the principal phagocytic cells involved in the ingestion and destruction of fungal pathogens. PMNs have potent fungicidal mechanisms and migrate through endothelial cell junctions to enter sites of inflammation in tissues. Dermatophytosis is caused by pathogenic fungi that have a preference for keratin-containing tissues, such as epidermis, nails and hair.
A logical classification of dermatophytosis is based on the site of infection, e.g. tinea corporis (skin), tinea capitis (scalp) and onychomycosis (nails). Microsporum, Trichophyton and Epidermophyton species are the most common fungal causes of dermatophytosis.2-4 In spite of the superficial nature of dermatophyte infections, haematogenous spread of the fungus or its antigens may occur that sensitizes the immunocompetent host and induce an immune response.5 Dermatophytes produce skin alterations in humans and other animals, and the essential role of the CMI response is to destroy the fungi and produce an immunoprotective status against re-infection. The resolution of the disease is associated with a delayed hypersensitive response.6 This work aimed at identification of the fungal agents from patients suffering from dermatophytosis, and detection of the effect of the isolated dermatophytes on PMNs and monocytes chemotaxis from healthy donors.
Study group
Fifty-three patients with superficial fungal infections of glabrous skin attended the Dermatology and Venereology out-patient clinic of Al-Hussien University Hospital, were studied during a period from May 2000 to September 2001. They were 28 females their age ranged from 5-55 (25.3±14) years and 25 males their age ranged from 3-66 (30.2±16.6) years old. A full history was taken from all patients including age, job, site of infection, presence of predisposing factors and antifungal treatment. The duration of the disease in 51 patients varied from 112 weeks (recent infection). In the remaining two patients, the duration of infection was more than 12 months (chronic infection). The patients were suffering from superficial dermatophytosis; no other skin infections or systemic diseases were detected.
Mycological examination was done for all patients. Antifungal sensitivity testing and chemotaxis assay were done for positive cultures. All of the recent cases in this study attended the out-patient clinic for the first time (not receiving either topical or systemic treatment).The two chronic cases were under irregular courses of antifungal therapy which was stopped for 15 days before the study.
Media used
Bufferes
Chemicals
Blood samples
Peripheral venous blood samples from apparently healthy adult volunteers (Age 18-45 years old) were collected in tubes coated with Lithium Heparin anticoagulant (Vacutte-Austria). Fresh human normal serum as a source of complement.
Collection of samples (Cutaneous Scales)
The circinate patches clinically suspected of harbouring a fungal infection were carefully cleaned with cotton swab impregnated with 70 % ethyl alcohol. Firmly adhering scales were then removed from the edge of the diseased area with a sterile scalpel and collected in a sterile container. A part of scales was used for potassium hydroxide (KOH) preparation and the other one was inoculated onto SDA medium at 26°C for up to 3 weeks.
Direct microscopic examination
Few drops of a 10 % of KOH were placed on clean glass slide. The material to be examined (scales) was added to it and a cover slip was then placed over this preparation. A brief, gentle warming over a Bunsen flame (avoid boiling) was done. The preparation was left for about 20-30 minutes, and then examined microscopically with low and high power of the microscope for pseudohyphae and arthrospores.
Isolation of the dermatophytes
A fragment of the scales was inoculated on SDA medium and incubated at 26°C for a maximum of 3 weeks or until growth and sporulation become visible. Diseased keratinous tissue harbors many organisms; therefore antibiotics such as penicillin at a concentration of 2000 ppm/L and streptomycin at a concentration of 40 mg/L were mixed with the growth media after autoclaving to avoid bacterial growth. Also, antifungal such as cycloheximide at a concentration of 500 mg/L was added to prevent other fungal growth. The purified fungal strains were subjected for identification and maintained on SDA at 4°C.8
Identification and speciation of isolated dermatophytes
The colonies were examined for the rate of growth shape, pigmentation and surface. Back of the colonies was examined for the presence of pigment and its colour.
Slide preparation was made and examined through an image analysis system (analysis soft imaging system Gmbh - Germany). Data were analyzed using the Regional Center for Mycology and Biotechnology (RCMB) Fungal Identification Database Management Software to demonstrate the presence of: hyphae, macroconidia, microconidia, chlamydospores and other fungal structures.
Growing of the fungal isolates on broth medium
Fungal isolates were subcultured on Sabouraud’s dextrose broth supplemented with 500 mg cyclohexamide and 50 mg chloramphenicol/L. Sixty ml of Sabouraud dextrose broth were distributed in 250 ml Erlenmeyer flasks each. Incubation was carried out at 25°C for a maximum 4 weeks or until growth and sporulation become visible. The mycelium was harvested and filtered under aseptic condition using Whatman filter paper No. 3. The mycelium was washed with sterile distilled water and transferred aseptically to be lyophilized.
Maxi Dry plus lyophilizer (Heto-Rotary Vane Pump-Vakkwumtechnik-Denmark) was used for lyophilization. Mycelium was subjected to cooling with 1 m bar negative pressure for 12 hours until freeze dried. Under aseptic condition, freeze dried mycelium was ground in a mortar until powdered.
Preparation of different combinations of lyophilized mycelium
For each organism, various combinations of the powdered mycelium in HBSS with or without fresh or heat – inactivated normal serum were prepared. The following combinations (C1-C8) were tested for their chemotactic activity:
C1 = 0.005 mg powdered mycelium + 5 ml HBSS.
C2 = 0.05 mg powdered mycelium + 5 ml HBSS.
C3 = 0.5 mg powdered mycelium + 5 ml HBSS.
C4 = 5 mg powdered mycelium + 5 ml HBSS.
C5 = 5 mg powdered mycelium + 0.5 ml fresh serum up to 5 ml HBSS.
C6 = 5 mg powdered mycelium + 0.5 ml heat inactivated serum up to 5 ml HBSS.
C7 = Negative control = HBSS which is not itself chemotactic.
C8 = Positive control = freshly prepared Zymosan Activated Serum (ZAS) up to 5 ml HBSS.
Preparation of ZAS
5 mg Zymosan were added to 1 ml fresh normal human serum. The mixture was incubated for 30 min. at 37°C. This induces generation of the C5a chemotactic fragment supernatant was used after centrifugation at 800rpm for 10 min.
Blood Sampling
Peripheral venous blood samples were taken from apparently healthy donors. Blood samples were collected in tubes coated with Lithium Heparin anticoagulant.
Leukocytes separation
Double separation of the cells without delay was carried out by the continuous density gradient centrifugation technique using two types of Histopaque9 as follows (Figure 1).
Two ml of Histopaque-1119 were placed into a 10 ml conical centrifuge tube. Another 2 ml of Histopaque–1077 were layered onto the Histopaque-1119. 4 ml of whole heparinized blood were added to the upper gradient (Histopaque-1077) of the tube. The tubes were centrifuged at 2300 rpm (700xg) for 30min. at room temperature (18-26°C) using general laboratory centrifuge. Centrifuge tubes were carefully removed. The top layer of plasma was gently aspirated using a plastic Pasteur pipette (Figure 1).
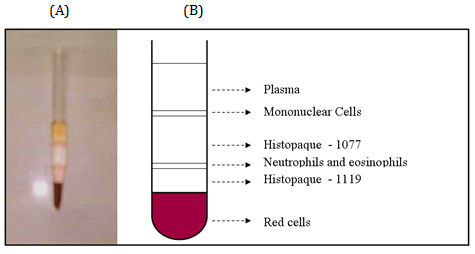
Figure 1 Centrifuge tube (A) and its schematic representation (B) showing the mononuclear and granulocyte layers on top of the upper and lower layers; respectively with plasma on the uppermost and RBCs on the lowermost tops.
Two distinct opaque layers were observed. Mononuclear cell series and platelet were found at the plasma/1077 interface (upper layer) whereas cells of the granulocytic series were found at the 1077/1119 interface (lower layer). Plasma was aspirated and discarded to within 0.5 cm of the upper layer. The mononuclear cells that were located at the upper interface were harvested and transferred to a tube marked (mononuclear cells). Fluid within 0.5 ml of the lower layer was aspirated and discarded. The polymorphonuclear cells (neutrophils) that were arrested at the lower interface were harvested and transferred to a tube marked (PMNs). Cells were washed by addition of 10 ml HBSS to the tubes and centrifuged for 10 min. at 800 rpm (200xg). Supernatant was removed and discarded.
Cells were re-suspended by gentle shaking, aspiration and addition of 10 ml PBS. Washing and resuspension were carried out twice. Following the last wash, cells were suspended in 1 ml of standard tissue culture (TC) medium RPMI 1640 buffered at pH 7.3 with HEBES buffer.
Monocyte isolation by adherence
Mononuclear cells were suspended in RPMI 1640 with serum at a concentration of 5 x 106 cells/ml. Forty ml of the suspension were immediately plated on 140 mm glass dish (2x108 cell /140- mm dish) and incubated at 37°C for 90 min. Non-adherent cells (lymphocytes) were pipetted off. The adherent cells (monocytes) were quickly washed (7-8 times) with preheated RPMI 1640 (37°C). After the last wash monocytes were suspended in separate RPMI 1640 and incubated at 37°C.
Assessment of viability by Trypan Blue Dye exclusion test
1:10 dilution of trypan blue in cell suspension was performed as follows:
Cell Counting
Simultaneously with the above procedure, cell counting was performed as follows:
Under agarose assay (Nelson Technique)
The assay was done according to Nelson et al.10 Eight clean glass slides were dipped in 0.5 % gelatin, rinsed in distilled water and left to dry by draining in air. Gelatin as a protein is requiring for optimal chemotaxis under agarose.
2 % Agarose and 0.5 % gelatin were dissolved in isotonic saline in a boiling water bath. Agarose was left to cool up to 48ºC. One volume of HBSS- HEPES buffer per volume of agarose was added giving a final concentration of 1 % agarose. 5 ml agarose were poured onto the gelatin coated slides and allowed to set at 4°C for 30 min. Figure 2 (A).
3 wells, 2.5 mm in diameter each, were cut in the agarose 2.5 mm apart Figure 2 (B). To the central well, 10μl of the cell type under testing were added (either PMNs or monocytes). To one of the two outer wells, 10μl of one of the eight test combinations (mentioned above) were added. To the other outer well, 10μl of HBSS -HEBES buffer (control solution) were added.
The slides were incubated at 37°C for two hours. Cells were then fixed in absolute alcohol for 30 min., which was replaced by fresh absolute alcohol and left overnight. The cells were finally stained with Harris’s Heamatoxylin for 5 min. The distances reached by the leading front of the cells in the direction of both the test substance and control in the line joining the centers of the wells were measured using the image analysis system (analysis Soft Imaging System GmbH-Germany) (Figure 3).
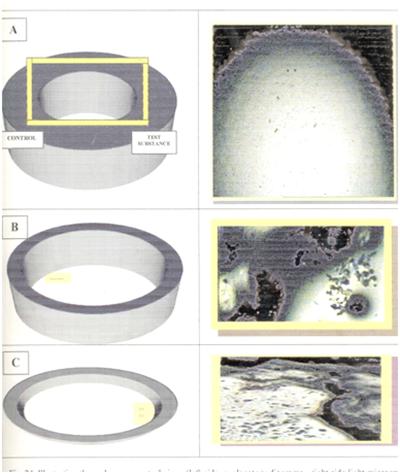
Figure 3 Illustrating the under-agarose technique (left side, explanatory diagrams-right side light micrographs for chemotaxis-reactive cells-X40).
Chemotaxis is the distance in mm that cells have migrated towards the test substance i.e, induced migration (normal average, 0.6-1.8 mm toward ZAS). Spontaneous migration is the migration distance towards the control (normal average, 0.2-0.4 mm). LI=induced migration / spontaneous migration.
Statistical analysis
Spss program version (10) was used for one way Anova statistical analysis. Pvalue was considered significant if it less than 0.05 and non significant if it was more than 0.05.11 LSD (least Significant Difference) values at 5% were determined.12
N.B: The difference between both values is considered significant if it is more than the value of LSD at 5%, non significant if it is less than this value. In this study, the differences between the LI of neutrophils and monocytes at each combination (C1, C2,…C6) were calculated by deducing the values of both cells at each combination and comparing the differences with LSD at 5%. Also, the LIs of each cell migrated towards each combination (C1-C6) was deduced from LSD at 5% of negative (C7) and from the positive controls (C8). The difference in either case was compared with LSD of related control.
Isolation of dermatophytes
Distribution of dermatophytosis in the patients group: The present study included 53 patients with superficial dermatophytosis. Clinical examination showed T. corporis in 14 (26.6%), T. versicolor and T. pedis in 24 (22.6%, each), T.cruris in 12 (18.8%) and T. capitis in 5 (9.4%) of these patients were complaining from Table 1.
|
Diagnosis |
T.pedis |
T.corporis |
T.capitis |
T.versicolor |
|
Patients’ numbers and % |
12 (22.6) |
10 (18.9) |
5 (9.4) |
12 (22.6) |
Table 1 Number and percentage (%) of patients with dermatophytosis according to their anatomical distribution (Total number = 53)
Nelson technique: In this assay the migration distances of two phagocytic cells namely monocytes and neutrophils, towards both the control (spontaneous migration, SM) and the tested fungal concentration (induced migration, IM) were recorded. As mentioned before, the leukotactic index (LI) of each cell = IM / SM.
Comparison between neutrophils and monocytes chemotaxis
The SM, IM and LI of neutrophils and monocytes towards M. canis are presented in Table 2 & 3. The LIs of neutrophils and monocytes migrated towards M.canis are presented in Table 4 and Figure 4. A significant difference between both cells has been found at C2-C6 and a non significant difference at C1 only. By comparing the combination of each cell with its negative and positive control, a significant difference has been found in neutrophil chemotaxis at C2, C3, C4 and C5 and a non significant difference has been found with C1 and C6, if compared with negative control. Again, significant difference has been found at all combinations when compared with positive control. A significant difference has been found in monocyte chemotaxis at all combinations, if compared with positive control. Comparing combinations with negative control, a significant difference has been found at C2, C4 and C6 and non significant difference was found at C1, C3 and C5.
|
Test Substance |
Spontaneous Migration (µm) |
Induced Migration (µm) |
Leukotactic Index |
|
C1 |
794.7 |
1027.04 |
1.29 |
|
C2 |
1316.26 |
833.14 |
0.63 |
|
C3 |
831.78 |
1356.72 |
1.63 |
|
C4 |
1301.6 |
937.52 |
0.72 |
|
C5 |
719.45. |
976 .00 |
1.35 |
|
C6 |
659.7 |
836.5 |
1.26 |
|
C7 |
946.3 |
976.08 |
1.03 |
|
C8 |
720 |
2184.14 |
3.03 |
Table 2 The measurement of the migration distances in µm recorded for neutrophiles when tested against C1-C8 combinations of M. canis using the under agarose technique
|
Test Substance No. |
Spontaneous Migration (µm) |
Induced Migration (µm) |
Leukotactic Index |
|
C1 |
562.25 |
746.1 |
1.3 |
|
C2 |
1040.4 |
880.4 |
0.8 |
|
C3 |
771.6 |
1020.6 |
1.3 |
|
C4 |
1225.8 |
575.05 |
0.4 |
|
C5 |
802.8 |
896 |
1.1 |
|
C6 |
605.2 |
842.4 |
1.4 |
|
C7 |
869.4 |
1108 |
1.2 |
|
C8 |
780.00 |
1673.1 |
2.1 |
Table 3 The measurement of the migration distances in µm recorded for monocytes when tested against C1-C8 combinations of M. canis using the under agarose technique
|
Combination |
Neutrophils |
Monocytes |
LSD At 5% |
Diff.* |
Sig. |
||
|
C1 |
1.29 |
1.3 |
0.04 |
0.01 |
Non S |
||
|
- ve** |
+ ve*** |
- ve |
+ ve |
||||
|
0.26 (Non S.) |
1.74 (Sig.) |
0.1 (Non S.) |
0.8 (Sig.) |
||||
|
C2 |
0.63 |
0.8 |
0.02 |
0.17 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.4 (Sig) |
2.4 (Sig) |
0.4 (Sig) |
1.3 (Sig) |
||||
|
C3 |
1.63 |
1.3 |
0.14 |
0.33 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.6 (Sig) |
1.4 (Sig) |
0.1 (Non Sig) |
0.8 (Sig) |
||||
|
C4 |
0.72 |
0.4 |
0.23 |
0.32 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
1.75 (Sig) |
2.31 (Sig) |
0.8 (Sig) |
1.7 (Sig) |
||||
|
C5 |
1.35 |
1.1 |
0.08 |
0.25 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.32 (Sig) |
1.68 (Sig) |
0.1 (Non Sig) |
1.0 (Sig) |
||||
|
C6 |
1.26 |
1.4 |
0.05 |
0.14 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.23 (Non Sig) |
1.77 (Sig) |
0.2 (Sig) |
0.7 (Sig) |
||||
|
C7 |
1.03 |
1.2 |
0.01 |
0.17 |
Sig. |
||
|
C8 |
3.03 |
2.1 |
0.01 |
0.93 |
Sig. |
||
|
LSD at 5% (C7) LSD at 5% (C8) |
0.27 |
0.11 0.25 |
|||||
Table 4 The LI of neutrophils, monocytes and LSD at 5% towards M.canis
Sig.= Significant
Non Sig.=Non Significant
*Difference between neutrophils and monocytes.
**Difference from negative control (C7).
***Difference from positive control (C8).
The SM, IM and LI of neutrophils and monocytes towards Trichophyton rubrum are presented in Table 5 & 6.
|
Test Substance No. |
Spontaneous Migration (µm) |
Induced Migration (µm) |
Leukotactic Index |
|
C1 |
954.24 |
1089 |
1.14 |
|
C2 |
550 |
870 |
1.58 |
|
C3 |
1124.16 |
1353.99 |
1.2 |
|
C4 |
705 |
720 |
1.02 |
|
C5 |
605.08 |
640 |
1.05 |
|
C6 |
1106 |
1294 |
1.16 |
|
C7 |
705 |
720 |
1.02 |
|
C8 |
402.9 |
648.44 |
1.6 |
Table 5 The measurement of the migration distances in µm recorded for neutrophils when tested against C1-C8 combinations of Trichophyton rubrum using the under agarose technique
|
Test Substance No. |
Spontaneous Migration |
Induced Migration (µm) |
Leukotactic Index |
|
C1 |
848.9 |
1185.25 |
1.4 |
|
C2 |
858.34 |
1035.9 |
1.2 |
|
C3 |
1185.3 |
727.86 |
0.6 |
|
C4 |
779.4 |
561.95 |
0.7 |
|
C5 |
692.05 |
764.46 |
1.1 |
|
C6 |
508.24 |
680.4 |
1.3 |
|
C7 |
832.86 |
909.3 |
1.09 |
|
C8 |
799.38 |
1181.04 |
1.47 |
Table 6 The measurement of the migration distances in µm recorded for monocytes when tested against C1-C8 combinations of Trichophyton rubrum using the under agarose technique
The LIs of neutrophils and monocytes migrated towards T.rubrum are presented in Table 7 and Figure 5. A significant difference between both cells has been found at C2, C3, C5 and C6 and a non significant difference at C1 and C4. Except for C2, a significant difference has been found in neutrophil chemotaxis at C1, C3, C4, C5 and C6 when compared with positive control. Again except for C4 and C5, a significant difference was found at C1, C2, C3 and C6. A significant difference was found in monocyte chemotaxis at C2, C3, C4 and C5 except for C1 and C6, when compared with positive control. By comparing with its negative control, a significant differences have been found at C1, C3, C4 and C6 except for C2 and C5. The SM, IM and LI of neutrophils and monocytes towards Trichophyton verrucosum are presented in Table 8 & 9.
|
Combination |
Neutrophils |
Monocytes |
LSD at 5% |
Diff.* |
Sig. |
||
|
C1 |
1.14 |
1.4 |
0.27 |
0.26 |
Non S. |
||
|
- ve** |
+ ve*** |
- ve |
+ ve |
||||
|
0.12 (sig.) |
0.46 (sig.) |
0.31 (sig.) |
0.07 Non Sig. |
||||
|
C2 |
1.58 |
1.2 |
0.23 |
0.38 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.56 (sig.) |
0.02 (Non sig.) |
0.11 (Non sig.) |
0.27 (sig.) |
||||
|
C3 |
1.2 |
0.6 |
0.18 |
0.6 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.18 (sig.) |
0.4 (sig.) |
0.49 (sig.) |
0.87 (sig.) |
||||
|
C4 |
1.02 |
0.7 |
0.31 |
0.32 |
Non S. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0 (Non sig.) |
0.58 (sig.) |
0.39 (sig.) |
0.77 (sig.) |
||||
|
C5 |
1.05 |
1.1 |
0.22 |
0.05 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.03 (Non sig.) |
0.55 (sig.) |
0.01 (Non sig.) |
0.37 (sig.) |
||||
|
C6 |
1.16 |
1.3 |
0.09 |
0.14 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.14 (sig.) |
0.44 (sig.) |
0.21 (sig.) |
0.17 (Non sig.) |
||||
|
C7 |
1.02 |
1.09 |
0.02 |
0.07 |
Sig. |
||
|
C8 |
1.6 |
1.47 |
0.07 |
0.13 |
Sig. |
||
|
LSD at 5% (C7) LSD at 5% (C8) |
0.1 0.16 |
0.14 0.18 |
|||||
Table 7 The LIs of neutrophils, monocytes and LSD at 5% towards T.rubrum
Sig.=Significant
Non Sig.=Non Significant
*Difference between neutrophils and monocytes.
|
Test Substance No. |
Spontaneous Migration (µm) |
Induced Migration (µm) |
Leukotactic Index |
|
C1 |
933.8 |
1101.69 |
1.17 |
|
C2 |
1007.2 |
1226.8 |
1.2 |
|
C3 |
649.15 |
717.72 |
1.1 |
|
C4 |
830.7 |
989.84 |
1.19 |
|
C5 |
737.5 |
893.48 |
1.2 |
|
C6 |
811.92 |
1059.12 |
1.3 |
|
C7 |
992.67 |
1261.2 |
1.27 |
|
C8 |
950.9 |
1289.9 |
1.37 |
Table 8 The Measurement of the migration distances in µm recorded for neutrophils when tested against C1-C8 combinations of Trichophyton verrucosum using the under agarose technique
|
Test Substance No. |
Spontaeous Migration (µm) |
Induced Migration (µm) |
Leukotactic Index |
|
C1 |
441.4 |
578.8 |
1.3 |
|
C2 |
752.76 |
906.56 |
1.2 |
|
C3 |
934.01 |
1075.5 |
1.15 |
|
C4 |
639.35 |
700.26 |
1.09 |
|
C5 |
826.56 |
893.27 |
1.08 |
|
C6 |
1022.88 |
1273.95 |
1.2 |
|
C7 |
919.31 |
1031.84 |
1.12 |
|
C8 |
968.87 |
1199.2 |
1.23 |
Table 9 The Measurement of the migration distances in µm recorded for monocytes when tested against C1-C8 combinations of Trichophyton verrucosum using the under agarose technique
The LIs of neutrophils and monocytes migrated T.verrucosum is presented in Table 10 and Figure 6: A significant difference between both cells at C1, C4, C5 and C6 and a non significant difference at C2 and C3. A significant difference has been found in neutrophil chemotaxis at all combination except for C6, when compared with positive control. By comparing it with negative control, a significant difference has been found only at C1 and C3. However, non significant differences have been found in monocyte chemotaxis at all combinations, for positive control. Again non significant differences have been found for negative control, except for C1. The SM, IM and LI of neutrophils and monocytes towards Trichophyton mentagrophytes are presented in Table 11 & 12.
|
Combinations |
Neutrophils |
Monocytes |
LSD at 5% |
Diff.* |
Sig. |
||
|
C1 |
1.17 |
1.3 |
0.11 |
0.13 |
Sig. |
||
|
- ve** |
+ ve*** |
- ve |
+ ve |
||||
|
0.1 (sig.) |
0.2 (sig.) |
0.18 (sig.) |
0.07(Non sig.) |
||||
|
C2 |
1.2 |
1.2 |
0.1 |
0 |
Non S. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.07(Non sig.) |
0.17 (sig.) |
0.08(Non sig.) |
0.03(Non sig.) |
||||
|
C3 |
1.1 |
1.15 |
0.16 |
0.05 |
Non S. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.17 (sig.) |
0.27 (sig.) |
0.03(Non sig.) |
0.08(Non sig.) |
||||
|
C4 |
1.19 |
1.09 |
0.09 |
0.1 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.08 (Non sig.) |
0.18 (sig.) |
0.03(Non sig.) |
0.14(Non sig.) |
||||
|
C5 |
1.2 |
1.08 |
|||||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.07(Non sig.) |
0.17 (sig.) |
0.04(Non sig.) |
0.15(Non sig.) |
||||
|
C6 |
1.3 |
1.2 |
0.07 |
0.1 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.03(Non sig.) |
0.07(Non sig.) |
0.08(Non sig.) |
0.03(Non sig.) |
||||
|
C7 |
1.27 |
1.12 |
0.12 |
0.15 |
Sig. |
||
|
C8 |
1.37 |
1.23 |
0.1 |
0.14 |
Sig. |
||
|
LSD at 5% (C7) LSD at 5% (C8) |
0.09 |
0.14 |
|||||
Table 10 The LIs of neutrophils, monocytes and LSD at 5% towards T. verrucosum
Sig.=Significant
Non Sig.=Non Significant
*Difference between neutrophils and monocytes.
**Difference from negative control (C7).
***Difference from positive control (C8)
|
Test Substance No. |
Spontaneous Migration (µm) |
Induced Migration (µm) |
Leukotactic index |
|
C1 |
794.7 |
952 |
1.1 |
|
C2 |
592.15 |
669.36 |
1.13 |
|
C3 |
969.29 |
1132.38 |
1.16 |
|
C4 |
1030.08 |
1157.7 |
1.12 |
|
C5 |
574.64 |
651.7 |
1.13 |
|
C6 |
764.1 |
934.15 |
1.22 |
|
C7 |
909.02 |
1146.33 |
1.26 |
|
C8 |
1208.7 |
1563.43 |
1.29 |
Table 11 The measurement of the migration distances in µm recorded for neutrophils when tested against C1–C8 combinations of Trichophyton mentagrophytes using the under agarose technique
|
Test Substance No. |
Spontaneous |
Induced Migration (µm) |
Leukotactic Index |
|
C1 |
588.2 |
716.82 |
1.2 |
|
C2 |
889.5 |
1108 |
1.24 |
|
C3 |
819.84 |
1025.68 |
1.25 |
|
C4 |
1159.02 |
1393.37 |
1.2 |
|
C5 |
874.9 |
1038.2 |
1.19 |
|
C6 |
1124.16 |
1353.98 |
1.2 |
|
C7 |
1085.96 |
1283.4 |
1.18 |
|
C8 |
929.53 |
1174.08 |
1.26 |
Table 12 The measurement of the migration distances in µm recorded for monocytes when tested against C1–C8 combinations of Trichophyton mentagrophytes using the under agarose technique
The LIs of neutrophils and monocytes migrated T.mentagrophytes was presented in Table 13 and Figure 7. A significant difference between both cells has been found at C1 and C4 and a non significant difference at C2, C3, C5, and C6. A significant difference has been found in neutrophil chemotaxis at all combinations except for C6, when compared with both positive and negative controls. No significant differences have been found in monocyte chemotaxis at all combinations except for C1, C5 and C6, if compared with positive control. Again significant differences have been found at C2 and C3 when compared with negative control.
|
Combinations |
Neutrophiles |
Monocytes |
LSD at 5% |
Diff.* |
Sig. |
||
|
C1 |
1.1 |
1.2 |
0.07 |
0.1 |
Sig. |
||
|
- ve** |
+ ve*** |
- ve |
+ ve |
||||
|
0.16(sig.) |
0.19(sig.) |
0.02(Non sig.) |
0.06 (sig.) |
||||
|
C2 |
1.13 |
1.24 |
0.1 |
0.11 |
Non Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.13(sig.) |
0.16(sig.) |
0.06(sig.) |
0.02(Non sig.) |
||||
|
C3 |
1.16 |
1.25 |
0.14 |
0.09 |
Non Sig |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.1 (sig.) |
0.13 (sig.) |
0.07 (sig.) |
0.01(Non sig.) |
||||
|
C4 |
1.12 |
1.2 |
0.06 |
0.08 |
Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.14(sig.) |
0.17(sig.) |
0.02(Non sig.) |
0.06(Non sig.) |
||||
|
C5 |
1.13 |
1.19 |
0.07 |
0.06 |
Non Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.13(sig.) |
0.16(sig.) |
0.01(Non sig.) |
0.07 (sig.) |
||||
|
C6 |
1.22 |
1.2 |
0.07 |
0.02 |
Non Sig. |
||
|
- ve |
+ ve |
- ve |
+ ve |
||||
|
0.04(Non sig.) |
0.07(Non sig.) |
0.02(Non sig.) |
0.06 (sig.) |
||||
|
C7 |
1.26 |
1.18 |
0.06 |
0.08 |
Sig. |
||
|
C8 |
1.29 |
1.26 |
0.11 |
0.03 |
Sig. |
||
|
LSD at 5% (C7) LSD at 5% (C8) |
0.08 |
0.05 |
|||||
Table 13 The LIs of neutrophils, monocytes and LSD at 5% towards T.mentagrophytes
Dermatophytes are a specialized group of fungi which affect keratinous tissue of humans and of other vertebrates, causing superficial infections.
In immunocompromised hosts, dermatophytes can directly invade deep dermal and subcutaneous tissues, and cause granulomatous or suppurative infections. The chronic localised superficial dermatophyte infection is the probable source of the deep infection elsewhere. It has been reported that dermatophytes can disseminate to internal organs including lymph nodes, lymphatics, bones, the liver, spleen and even the brain in very rare instances.13 In the current work ermatophytes were the most common pathogens recovered from our patients with dermatomycoses. In our study, identification and classification of the M.canis, T.rubrum, T.verrocosum and T. mentagrophytes isolates were based mainly on themacroscopic inspection of colony morphology andanalysis of their microscopic characteristics.
The infection can arise at any age; with most cases occurring during adolescence and young adulthood. Hormonal changes or increases in sebum secretion might be relevant.14 The current study revealed that the incidence of the disease had increased with age which was more in the group of >20 years than those < 20 years (P > 0.05). However, no significant difference, this may be due to the fact that with aging patients become more exposed to diseases which may suppress their immune system as diabetes, autoimmune diseases, tumors. etc., which are risk factors for mycoses. Exception of this finding was detected with T. capitis and T. cruris. This supported by the work of Wright S, Robertson VJ15 who found it in classmates of children with Tinea capitis.
In the present study, the rate of dermatophytosis in the female group was higher than that in the male group with the ratio1.2: 1.00, with no statistically significant differences (P> 0.05) found between either sex, age in one hand and duration of illness, clinical diagnosis and cultural diagnosis on the other hand. Our study showed that in most of the tinea cases the male: female ratio was quite similar, except for Tinea pedis (Female > males) and in tinea versicolor (Males > females). Welsh et al.16 reported that most of the cases were equally distributed in both genders, except for Tinea cruris which was more prevalent in men (3.5: 1 ratio). In the present study, the clinical diagnosis of 53 patients showed that 26.6% of patients were suffering from T.corporis followed by 22.6% with both T.pedis and T.versicolor, 18.9% of patients with T.cruris and Tinea capitis in only (9.4%). The same results were reported by Dolenc-Voljcˇ,17 who revealed a lower percentage of Tinea capitis cases (3.9%) than in other countries with high prevalence of M. canis infection, where Tinea capitis reportedly accounted for 6-18%, or even for 36% of all dermatophyte infections. Also, Ellabib and Khalifa18 studied 3812 patients with dermatophytosis attending the dermatology clinic in Tripoli, Libya and found that they were 45.9% with T.corporis, 8.1% with T.pedis, and 27.8% with T.versicolor.
The range of adaptive mechanisms shown by fungi varies from changes in cell wall structure and width, the deposition of melanin, and capsule formation, to the production of toxins or biocides, and the elaboration of immunomodulatory substances; the existence of antigenic mimicry and antigenic variation seen with other micro-organisms has been reported in a few fungi and the convergence of complement and sterol receptor structures in mammalian and fungal cells may prove to be of protective advantage to fungi.19
Evaluation of the overall fungal chemotactic activity represents the mainstay of this work. Most of the tested dermatophyte concentrations were stimulatory for either neutrophils or monocytes. Cassone20 affirmed that in all diseases caused by the principal human pathogenic fungi, there is evidence that a more or less intense cell-mediated immune reactivity is generated in the normal or simply colonized host and that this is lost or greatly diminished when the host is markedly affected by the disease.
In the current study, except for two patients, the duration of illness was less than 12 weeks (recent infection). The two chronic cases were under treatment. This may help in understanding how the fungal antigens in these patients were still active in different concentrations. The present study is supported by Nickerson et al.21 and Gong et al.13 who stated that depression of in vitro cellular immune responses is commonly observed in progressive fungal infections.
Patients with persistent foot infections have been reported to show reduced lymphocyte blastogenesis or leukocyte migration inhibition to dermatophyte antigen.22,23 This is exemplified in the current study by the suppression of chemotactic activity of leucocytes (PMNs and monocytes) with increasing concentration of powdered mycelium of T. rubrum. Moreover, suppression was more obvious with monocytes. The ability of fungal cell wall antigens, mostly polysaccharides in nature, to down-regulate or suppress cell-mediated immunity has been well documented as a means of fungal invasion to the host immune system.20 It is possible therefore that these antigens may act as an immunomodulators.24 Furthermore, poly- and oligosaccharides can interfere with the recognition and binding of non-opsonised fungal particles by phagocytes,25 pointing to the possibility that cell wall material shed from hyphae might prevent phagocytes from attacking the fungus in tissue. Likewise chemotaxis may be inhibited in this case.
Other views suppose that the inhibitory activity of some fungi appears to reside in a fraction of cytoplasmic antigens.19 Fungal metabolites, e.g. gliotoxin, also share in the inhibitory effect of fungi on the biological activities of immune cells.26 El-Sheikh27 also found that grisofolvin suppress the lymocyte chemotactic activity. Depression of cellular immunity by the fungus may provide a permissive environment for progression in the host.28 Other metabolites like β-glucan originally identified as the component of zymosan responsible for macrophage activation and neutrophiles.29,30 β-glucan has been shown to stimulate hemopoietic immune effector cells, both in vivo and in vitro models.31
The experimental data recorded through Nelson techniques support the fact that M.canis exerted an invariable stimulator effect on all the tested leucocytes, namely, PMNs and monocytes. On the other hand T.verrocosum was the most stimulatory antigen tested. Neutrophiles were more actively migrating than monocytes. A significant difference has been found between most of LIs of neutrophiles and monocytes migrated towards the four tested fungi,as the differences between LIs of these cells were more than LSD at 5% (least significant difference). This can be explained, in part, by the report of Davies and that neutrophils are attracted to the site of fungal infections both by certain fungal cell wall or cytoplasmic antigens.
It is also known that monocytes came after neutrophiles in attacking microbial or other invaders. These finding is supported by the notion of Calderon and Hay23 that neutrophiles and to a lesser extent monocytes, can kill dermatophyte conidia. Moreover, Calderon and Shennan23 stated that this activity depends on both intra- and extracellular mechanism, and the generation of the respiratory burst is an important stage in this process.
Study of the dose-effect relationship in fungal chemotaxis has been focused upon in the present work. The relative effects of different concentrations of lyophilized mycelia on immune cells' migration were investigated by close monitoring of the leukotactic indices of the immune cells at various combinations of fungal elements viz. C1, C2, C3 and C4. Except for T.mentagrophytes with both cell types and T.rubrum with monocytes.The present data showed a non proportional dose-effect relationship between fungal concentration and leukotactic indices. Nevertheless, El-Sheikh et al.32 found that there is a directly proportional dose-effect relationship between fungal concentration and leukotactic indices, i.e. the higher the dose the more is the effect.
The current study has also analyzed the role of serum in mediating fungal chemotactic activity towards the immune cells. Comparing the leukotactic indices recorded at C5, where fresh serum was added to fungal elements, and at C6, where heat-inactivated serum was added instead, not revealed serum-mediated activity for lyophilized mycelia. However, El-sheikhet al.32 revealed minimum, if not at all, serum mediated activity for lyophilized mycelia of isolated dermatophytes. Serum suppressor factors on lymphocytes and neutrophiles were studied by different investigators.33 found an inhibitory effect of sera from leprosy and systemic lupus erythematous patients on neutrophil chemotaxis using zymosan as a fungal chemoattractant. The in vitro nature of the study may play a role in these results. Further, the dose of fungal elements capable of inducing serum activation may vary from one organism to the other according to the topographical features, the antigenic personality of each fungus and the tested leukocyte cell type. Other workers on chemotaxis like El-Sheikh et al.32 had used quantitative technique, i.e., Boyden chamber assay. However, they found that Nelson technique gave similar results.
Finally, Nelson technique is a qualitative measure allowing only simple judgment on the behavior of a bioeffector whether stimulatory or inhibitory from the chemotaxis point of view. On the other hand, topical and systemic antibiotic uses might have enhanced the chance of fungal infection. These factors might have precipitated superficial dermatophytes progressively invaded into the subcutaneous tissue and superficial lymph nodes, and finally came into being granuloma.34 Furthermore, haemolysins produced by Trichophyton species may similarly play an important role in balancing the host’s cellular immunity and the ability of the fungus to diminish the immune response. Haemolytic activity levels in dermatophytes have been shown to correlate with the severity and chronicity of clinical infection. Some of the extracellular enzymes such as keratinase, elastase, collagenase and lipase that diffuse through the cornified layer of skin during infection may allow persistence of fungi in skin and lead to chronicity and deeper infection.35-39
Most of the tested dermatophyte concentrations were stimulatory for either neutrophil or monocyte chemotaxis (LI> 1.0).40-42 These data stress on the important role of neutrophil and monocyte in host defense against dermatophyte infection. The data also indicate that neutrophil is a more active responder to fungal infection than monocyte. A non proportional dose-effect relationship between fungal concentration and leukotactic indices was observed. No significant role for serum in mediating chemotaxis was found in the studied species.

©2016 Mohamed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.