MOJ
eISSN: 2373-4442


Research Article Volume 6 Issue 5
1Ural Federal University, Russia
2Institute of Immunology and Physiology, Russia
3Regional Children Clinical Hospital, Russia
Correspondence: Svetlana Deriabina, Institute of Immunology and Physiology, Laboratory of Molecular Diagnostics, Ural Federal University, Russia
Received: January 01, 1971 | Published: September 24, 2018
Citation: Deriabina S, Bolkov M Tuzankina I, et al. Case of chromosome 22q11.2 deletion syndrome in Russian family. MOJ Immunol. 2018;6(5):134-138. DOI: 10.15406/moji.2018.06.00208
There is a family under our surveillance, wherein two siblings and their mother have the 22q11.2 deletion syndrome. It was found different clinic manifestation of genetic disorder with one type of mutation in one family in two generation. A family with the 22q11.2 deletion syndrome presented at two siblings and their mother was observed. Phenotypic manifestations in family members with 22q11.2 deletion syndrome were analysed and molecular genetics study was carried out. Conclusion that phenotypic features and size of chromosome 22q11.2 deletion may be different in family members was made. The further research is required to determine the deletion length and definition of its component genes, as well as to establish the genotype-phenotype interactions and disease prognosis.
Keywords 22q11.2 Deletion Syndrome, Immunologic Deficiency Syndromes, Phenotype, Siblings, conotruncal, hypoparathyroidism, turbidimetry, heart anomalies, phenotypic manifestations, thymic hypoplasia, facial dysmorphism, bacterial pneumonia, necrotizing colitis, serous peritonitis.
Our Center for Clinical Immunology has been working in the sphere of primary immunodeficiencies (PID) for 30 years. Early diagnostics and management of PID patients is our priority.1,2 Follow-up of adults and children in our Center can be done as it has two main clinical bases - Region Children Clinical Hospital № 1 and Region Clinical Hospital № 1 (Yekaterinburg, Ural region, Russia). Center also closely collaborates with the Institute of Immunology and Physiology of Russian Academy of Sciences and international organizations on PID - jproject (collaboration with Professor L. Marodi, University of Debrecen) and Jeffrey Modell Foundation. There are 15 patients with chromosome 22q11.2 deletion (14 children and 1 adult) out of total number of 309 PID patients in our Center for Clinical Immunology. Thereby, the prospects for identifying these patients are wide. According to many reports, chromosome 22q11.2 deletion occurs 1: 3,000 - 6,000 live births, affecting both sexes equally.3,4 It has been reported that chromosome 22q11.2 deletion is found in 90% of the patients with disgorge phenotype, 70% of the patients with Velo-Cardio-Facial Syndrome (VCFS), and 15% of the patients with isolated conotruncal cardiac defect.5 Most of the patients were diagnosed by slightly and severely decreased immunity, facial defects, and heart anomalies and some patients had kidney abnormalities, hypoparathyroidism, hypothyroidism, and developmental retardation.6 Chromosome 22q11.2 deletion is mostly diagnosed in early childhood by pediatricians as a congenital disease so that the syndrome is difficult to diagnose in the late adulthood7‒9 In literature there are only a few works devoted to adult patients with chromosome 22q11.2 deletion syndrome.3,4,10‒12 This is due to low awareness of physicians and other specialists of chromosome 22q11.2 deletion syndrome as well as high variability of phenotypic manifestations of this syndrome and the presence of mild forms. Under our surveillance there is a family K, wherein two siblings and their mother have the chromosome 22q11.2 deletion syndrome. The purpose of this study is to analyze phenotypic manifestations in family members with chromosome 22q11.2 deletion syndrome.
We assessed the clinical characteristics of the disease, medical history and family history of family members with chromosome 22q11.2 deletion syndrome, performed general examination, biochemistry, and immune tests, ultrasound scan of the heart, thymus, thyroid, and abdominal organs. Subsets of the lymphocytes were estimated by flow cytometry with staining by monoclonal antibodies (Beckman Coulter, USA). Serum immunoglobulin (iga, igm, igg) levels were determined by turbidimetry (automatic biochemical analyzer Cobas Integra 400, Hoffman-La Roche Ltd). Ultrasound examination was carried out on a stationary ultrasonic apparatus Vivid 7, General Electric Medical Systems (USA). An ultrasound examination of the heart, thymus, thyroid, abdominal organs was carried out. The whole blood was taken from the girl and her parents for molecular genetic testing. Boy's genetic material (dry blood spot) was obtained from the archives of Neonatal Screening Laboratories of Clinical Diagnostic Center "Mother and Child Health" (Yekaterinburg). DNA was isolated using a kit (qiaamp DNA Mini Kit (QIAGEN, Germany). DNA from dried blood spots was isolated using a commercial kit "DNA-sorb-B» (Amplisens, Russia). Multiplex ligation-dependent probe amplification (MLPA) set SALSA MLPA probemix P250-B2 digeorge (MRC-Holland, The Netherlands). The kit contained 48 different MLPA-probes, 29 of which covered the chromosomal region 22q11. The analysis was carried out according to the manufacturer instruction (MRC-Holland) in Genetic Analyzer Applied Biosystems 3500. For computer data processing, software Coffalyser (MRC-Holland) was used. Fluorescence in situ hybridization (FISH) was performed on peripheral blood lymphocytes of mother, father and daughter with probe XL TUPLE (LSI 22q11, LSI 22q13) (metasystems, Germany). The work was conducted in compliance with the principles of volunteerism and confidentiality, according to Fundamentals of Russian Legislation, on health care (22.07.1993 N 5487-1, ed. 07.12.2011) and the European Convention on Human Rights (1999-2000).
As with other microdeletion syndromes, del22q11.2 syndrome shows clinical polymorphism with predominance of such signs as congenital heart and trunk vascular defects, facial dysmorphism, deformations of the hard and soft palate, underdevelopment of thymus, delay in physical development, decrease in weight and height indices, delay of psychomotor development, hypoplasia of parathyroid glands, hypocalcemia, immunity disorders. However, phenotypic variants can be very diverse. This is illustrated by the family cases of this syndrome that we observed in which different manifestations of the same genetic pathology occurred within family. Thus, we recognized three cases of chromosome 22 deletion syndrome in family K. The mother and the daughter were diagnosed with so called mild disease phenotype. 8,9,11,15 Both patients are alive and the mother’s fertility is preserved. The third case in this family was the boy who had a more expressed phenotype (failure to thrive, congenital heart disease, hyperplasia of the thymus, features of combined immunodeficiency, operative intervention for correction of the heart defect, the inadequacy of reparative processes after surgery, postoperative sepsis, multiple episodes of viral infections and severe viral- bacterial pneumonia, necrotizing colitis and serous peritonitis in the end of life), leading to death. For the purpose of postmortem molecular genetic verification of the boy, we requested his dry blood spot from neonatal screening. In our country, the national PID screening program has not yet been launched, but in some cases we conducted our own studies to analyze the effectiveness of TREC/KREC assay. 16,17 The TREC/KREC assay in these patients could have lower diagnostic significance in compare with SCID. The majority of digeorge patients have TREC/KREC levels in the normal range .18 The genetic identification of chromosome 22q11.2 deletion syndrome in our work was carried out using the method of multiplex ligation PCR amplification (MLPA) which has a number of advantages over other methods: we can analyze up to 50 sequences from DNA sample simultaneously, need only 20 ng of DNA, can be used partly degraded samples .13,19,20 Moreover, the results are available within 24 hours, don’t depend on subjective analysis of an expert and the price.21,22 The application of MLPA method increased the possibility of molecular identification of chromosome 22q11.2 deletion, allowed to raise the diagnostic accuracy of sites involved in microdeletion and also enabled retrospective diagnosis.
The parents were not blood relatives in the described family (Figure 1). They grew up in different towns located at a great distance from each other. Information about father’s relatives (III.10) is not available because he grew up an orphan in the small town of Sverdlovsk region. At first we identified a boy with digeorge syndrome in the described family.
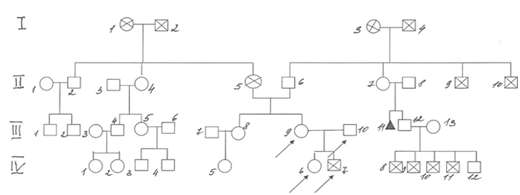
Figure 1 Family К. pedigree.
IV.3–boy 5 yo, Down syndrome and Infantile cerebral palsy
IV.5–woman 18 yo with recurrent furunculosis
IV.6–girl, 1 y. 3 m., with 22q11 deletion syndrome (22q11 DS)
IV.7–boy with 22q11 deletion syndrome, died at 8 m.
IV.8–IV.11 – 4 boys, died at the age of 1 year from unknown causes, were born and lived in one of the regions of Kazakhstan, known as a region of environmental problems, of a chemical-radioactive nature, of anthropogenic origin.
IV.12–man 22 yo, short stature (dwarfism).
III.8–woman 36 yo, sister of patient with 22q11 DS
III.9–woman 34 yo with 22q11 DS, mother of two child’s with 22q11 DS from described family
III.10–man 36 yo, father of child’s with 22q11 DS.
III.11–miscarriage in the 12 weeks of pregnancy
II.2–man 65 yo with congenital heart defect
II.4–woman 62 yo with diabetes mellitus.
II.5–a woman suffering from coronary heart disease, an atrioventricular blockade, a pacemaker installed, died of a stroke at age 65, the mother of a patient with 22q11 DS.
II. 9–II.10–died in the war.
I.1–woman died of a stroke at 83 years old.
I.2–man, died of stomach cancer in 60 years.
I.3–I.4–died at the age of over 60 for unknown reasons.
Son-first patient was observed in Regional Children Clinical Hospital (Yekaterinburg, Russia) with diagnosis: Primary Immunodeficiency - digeorge syndrome (DGS). Thymic hypoplasia. Сongenital heart disease: truncus arteriosus, DC 2b stage by Lang, corrected).The child was from I pregnancy, heart defect was set prenatally, marked growth retardation. Childbirth at 38 weeks, weight 1885g., length 43cm. A severe condition from birth, received therapy in preparation for surgery. Breastfeeding up to 1 month. The peripheral blood cells were normal in the dynamics (Table 1). The boy had hypogammaglobulinemia (iga - 0, igm - 1.25 g/l, igg - 1.6 g/l) and his blood calcium level was at the lower limit of normal value. These figures remained in the dynamics (Figure 2). The reduction of the thymus was found on ultrasound (mass 2g at the age of 1 month) as well as diffuse parenchymal changes of organ, weight 2 g. He was operated for congenital heart disease on 42 day of life. During the surgical intervention the thymus wasn't found in a typical position. The postoperative period was complicated by sepsis, heart and respiratory failure. He received intravenous immunoglobin (Intratekt) at 400 mg/kg, antibiotic therapy (Sulperazon, Maxipime, Timentin, Ciprofloxacin, Biseptolum) and fluconazole. At 5 months – severe upper respiratory infection and complication as respiratory failure type II. At 6,5 and 8 months episodes of lower respiratory infection. Died on the 9th month of life complicated with pneumonia, sepsis, peritonitis, necrotic colitis and brain edema. The diagnosis was confirmed on autopsy. In addition to clinically detected pathology, more heart injuries were detected: fibrosis and myxomatosis of single pulmonary valve, hypertrophy of both ventricles, subendocardial sclerosis of the right ventricle, hyperelastosis and sclerosis of the right ventricle, hyperelastosis and sclerosis of the pulmonary artery . Hypoplasia of adrenal glands was found as well and was confirmed an insufficiency of reparative abilities - residual defect of the lower interventricular septum. The microdeletion in starting region digeorge (LCR22-A) was indentified on the postmortem genetic study-from the 4-year-old dry blood sample.
|
1 Month |
2 Months |
Reference range |
WBC |
6.0*109/l |
5.6*109/l |
5.5-12.5*109/l |
Lymph % |
42 |
68 |
43.0-74.0 |
CD3+ lymphocytes % |
66 |
61 |
45.0-79.0 |
CD4+ lymphocytes % |
46 |
37 |
36.0-61.0 |
CD8+ lymphocytes % |
12 |
25 |
16.0-34.0 |
CD16+ lymphocytes % |
7 |
26 |
6.2-18.2 |
CD19+ lymphocytes % |
24 |
15 |
19.0-31.0 |
IgA, g/l |
0 |
0.1 |
0.1-0.4 |
IgM, g/l |
0.25 |
0.6 |
0.4-1.8 |
IgG, g/l |
1.6 |
3.5 |
1.2-12.8 |
Table 1 Clinical characteristics and laboratory analyses of boy during hospitalization at regional clinical children hospital No.1 in Ekaterinburg.
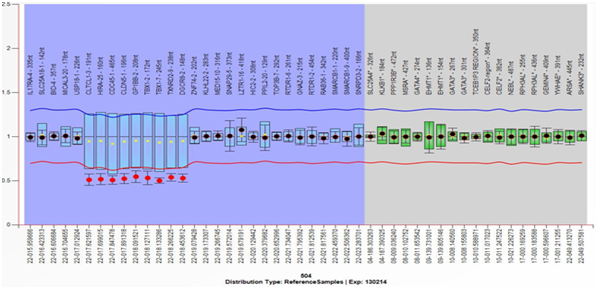
Figure 2 Multiplex Ligation-dependent Probe Amplification (MLPA) analysis result from the mother, showing the deletion of the 22q11.2 region and the genes included therein. Deletion is defined with a cut-off ≤7.
The girl, second child in the described family fell under our supervision in 1 year 10 months. She followed up with diagnosis: Primary Immunodeficiency - digeorge syndrome (DGS). Thymic hypoplasia. Congenital heart disease: atrial septal aneurysm, tricuspid regurgitation III, not operated. The child of II pregnancy with late gestosis, delivery at 34 weeks of gestation by caesarean section, had 3 months of respiratory support. Birth weight 2190 g, length 43 cm. Breastfeeded up to 3 months. Among the objective data, the first place is the dysmorphic features of face, which usually point on chromosome abnormality – the girl have hypertelorism, submandibulism and dolichocephaly. She has height and weight in healthy range, normal peripheral lymph nodes up to 0,5 cm in diameter (cervical, submandibular, axillary, inguinal). There was no murmur detected when examining the heart with a stethoscope. Normal respiratory and heart rates. Cardiac sounds are rhythmic, the heart rate is 98 beats per min. The abdomen is soft and painless. The sizes of the liver and spleen are within normal limits. Mild hypogammaglobulinemia was found (iga - 0 g/l, igm - 1.4 g/l, igg - 3.0 g/l). Thymus ultrasound (at age 1 month) showed a reduction of the thymus weight – not more 4 g. The results of common blood test at age 1 month were not remarkable: Leu 6,35*109/l; Lymph - 60% (3,81*109/l); СD3+ – 58% (2,21*109/l); СD4+ – 38% (1,45*109/l); СD8+ – 15% (0,57*109/l); CD4+/CD8+ – 2,53; СD16+ – 16% (0,61*109/l); СD19+ – 24% (0,91*109/l). Level of calcium in healthy range (2.5 mmol/l). After mild episodes of upper respiratory infections in 1 year 3 month common blood test and levels of immunoglobulins without significant changes: Leu – 7,70*109/L; Lymph - 67% (5,16*109/l); СD3+ – 73% (3,77*109/l); СD4+ – 49% (2.53*109/l); СD8+ – 22% (1,13*109/l); CD 4+/CD8+ - 2,23; СD16+ – 6% (0,31*109/l); СD19+ – 20% (1.03*109/l). Iga - 25 mg/dl, igm – 67 mg/dl, igg – 480 mg/dl. Now is constantly followed up by a pediatrician and an immunologist. The daughter’s genetic investigation revealed deletion in the starting area of the digeorge region (LCR22-A), including genes CLTCL1, HIRA, CDC45, CLDN5, GP1BB, TBX1, TXNRD2, DGCR8. Using FISH-method we confirmed a loss at 22q11.2 in daughter’s samples 46,XX.ish del(22)(q11.2q11.2)(TUPLE1)(HIRA).Mother-34 years. Was born in a town in Kazakhstan, located near the nuclear test area where nuclear devices have been tested for 50 years. Semipalatinsk refers to the zone of increased radiation risk (dose exposure of the population comprised from 7 to 35 Baer for the whole test period.13,14 She had a few incidents of pneumonia until she was 10 years and some upper respiratory infectious episodes, but not significant signs of immunodeficiency. She was diagnosed with Ullrich–Turner syndrome at the age of 10 based on growth retardation and failure to thrive. But the onset of puberty and fertility indicates the mistake of doctors (no genetic studies in childhood). The patient started sexual activity at the age of 25 with using mechanical contraception. She had two pregnancies with no abortions or infertility. Currently, she has the arterial hypertension (rise up to 150/90 mm Hg, adapted to the level of 140/90 mm Hg). Today she is having body mass index of 40.37 and some dysplastic features of the face: hypertelorism and pear-shaped nose, short neck. Peripheral lymph nodes are normal up to 1 cm in diameter. There was no murmur detected when examining the heart with a stethoscope. Cardiac sounds are rhythmic; heart rate is 68 beats per min. The size of the liver and spleen is within normal limits. Laboratory findings (at age 34 years): peripheral blood cells in normal range: Leu 9.2*109/l; Lymph-34% (3.13*109/l); СD3+ – 78% (2.44*109/l); СD4+ – 65% (2.03*109/l); СD8+ – 15% (0,47*109/l); CD4+/CD8+ – 4.33; СD16+ – 12% (0.38*109/l); СD19+ – 9% (0.28*109/l). Immunoglobulins are with tendency to increase iga: iga - 500 mg/dl, igm - 180 mg/dl, igg - 1400 mg/dl. Biochemical parameters within normal range: glucose-4.3mmol/l, Ca-2.3mmol/l, hba1c-5,7%. Heart ultrasound showed normal picture without any pathology, two solid cysts were found in thymus ultrasound and hepatic steatosis found in abdominal ultrasound. Genetic study revealed a deletion in the starting area of the digeorge region (LCR22-A), including genes CLTCL1, HIRA, CDC45, CLDN5, GP1BB, TBX1, TXNRD2, DGCR8. We confirmed a loss at 22q11.2 in samples of mother 46, XX.ish del(22)(q11.2q11.2)(TUPLE1(HIRA) using FISH method.
Father, 36 years, was born and grew up in the little town of Sverdlovsk region. He had varicella in childhood, and currently rare episodes of upper respiratory infections, no chronic diseases. He didn't have any signs of immunodeficiency or any abdominal or infection disease. He had normal count of leucocytes and immunoglobulins, normal calcium and glucose levels. In different ultrasound studies only a hepatic steatosis was found. And we did not found any microstructural changes in digeorge region in DNA.
None.
None.

©2018 Deriabina, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.