MOJ
eISSN: 2373-4442


Background: The aim of the study was to find the effect of hypobaric hypoxia (HH) on IgA and complement system activation in time-dependent manner.
Methods: Serum levels of IgA, C3, C3a, C5, C5a, SERPING1 and Complement Factor Properdin (CFP) were detected by Enzyme-linked immunosorbent assay in male Sprague Dawley rats under HH at 25,000 ft (7620m). SD rats were exposed for 1, 3, 7 and 14 days in animal decompression chamber and relation between control versus HH exposed rats was analyzed.
Results: Serum levels of immunoglobulin IgA were upregulated with HH exposure and complement proteins like C3, C5 (p<0.01) were found to be maximum on 14th day of exposure. Anaphylatoxin, C3a also upregulated significantly on14th day. C5a, another anaphylatoxin increased upto 7th day though not significantly. CFP was significantly down regulated with hypoxia exposure of 1 and 3 days and levels increased on further exposure. SERPING1 levels increased significantly on 14th day of exposure.
Discussion: Our study on rats revealed that hypobaric hypoxia alters the secretary IgA level and complement proteins in a time-dependent manner. Thus concluding that extreme environmental condition may alter the humoral immune response.
Keywords: hypoxia, complement proteins, IgA, stress, high altitude
An altitude above 8000 feet is considered as high altitude which includes oxygen-compromised environment. Such environment results in a condition called hypobaric hypoxia (HH) which may induce diseases/problems in different organ systems. Diseases like Acute Mountain Sickness (AMS), High Altitude Pulmonary Edema (HAPE), and High Altitude Cerebral Edema (HACE) occur commonly in natives, mountaineers and soldiers recruited at high altitude. While much work has been done on the effects of high altitude but its impact on immune system is less studied so far. Immune system is a network of different cells, tissues and organs which help in defending the body from foreign substances and other harmful stressors. It gets affected by extreme environments, invading harmful micro-organisms and disease conditions. It is now established that stressful conditions do affect the immune system. In some reports, high altitude environment has shown to affect different immune cells like T-cells, B-cells, NK cells and macrophages.1,2 Studies on harsh environment of Antarctica also suggested that stressful conditions of Antarctica lead to increased serum IgA levels.3 One of the important branches of immune system is the Complement system which helps the immune cells to get activated against the invading micro-organisms and different inflammatory molecules, thus provides a bridge between innate and adaptive immune response.4 Complement system is activated via three pathways namely classical, alternate and lectin.5 In many studies, Complement system has also been shown to get activated by different stressful environments. In a study, performed on human subjects who went to Antarctica, it has been shown that stressful conditions led to the activation of complement cascade. Factors like C3, C4, C3a, C4a, C5a and Complement Factor B were modulated in the summer as well as winter over expedition members.6 A Study on rats sensitive to hypoxia revealed activation of complement system components with different degrees of hypoxia exposure for maximum of 3 days.7 Also, levels of complement protein like C4 has been seen to alter on acute HH exposure8 HH is a stressful condition; human go to pilgrimages, mountains or works at such high altitude which results in immune compromisation. IgA has been seen to play a major role in defending to infections on mucous membranes. It has been seen that there is a higher incidence of infections in people going to high altitudes, therefore the study was done in an attempt to understand immunity of people at high altitude, changes in IgA levels and complement factors were studied. As in rats, pathological changes are majorly seen at an altitude of 25,000 ft,9 the rats were exposed to simulated hypobaric hypoxic conditions in specially designed decompression chamber. Our study revealed that HH significantly modulated the levels of IgA and complement factors in rats. The results can be applied to unfold mechanism of immune system activation in humans during high altitude (hypobaric hypoxia) stay.
Reagents
ELISA kits used for the study are as follows: Rat Complement C3a (Catalog No. MBS2503333), Rat Complement C3 (Catalog No. MBS724829), Rat Complement Factor Properdin {CFP} (Catalog No. MBS725540), Rat Plasma Protease C1 Inhibitor {SERPING1} (Catalog No. MBS910407), Rat Complement C5a (Catalog No. MBS760397), Rat Complement C5 (Catalog No. SL1301Ra) and Rat IgA (Catalog No. 8850480).
Experimental animals
Male Sprague-Dawley (SD) rats (220±20g) were bred in the IEAC (Institutional Ethical Committee on Animal Experimentations) approved animal facility of Defence Institute of Physiology and Allied Sciences (DIPAS), Delhi. The animals for study were approved by animal ethical committee of the institute in accordance with the guidelines of “Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA)” of Govt. of India. The animals were maintained under pathogen free conditions on bedding of rice husk in polypropylene cages and were fed with standard rodent pellet and water ad libitum in the institute’s animal facility at 25±1◦C, 55±10% humidity and 12-h light-dark cycle. All experiments were performed in accordance with the regulations specified by the IEAC and conformed to National Guidelines on the care and use of Laboratory animals, India. The disposal of experimental animals is strict comply with the management requirements of experimental animals.
Animal exposure to simulated HH
Thirty animals were randomly divided into 5 groups of six animals and groups were assigned names as Control, 1 Day, 3 Days, 7 Days and 14 Days. Animals of Control group were kept in normal environment without HH exposure, whereas animals of other 4 groups were exposed to simulated HH conditions in a animal decompression chamber maintained at a pressure of 282 torr which corresponds to an altitude of 7620 m for continuous exposure of 1, 3, 7 and 14 Days respectively. The rats were exposed continuously for respective days with 15 min interval once/day for food, water and cage replenishment. Continuous supply of fresh air at rate of 8L/min was provided to prevent accumulation of carbon dioxide. The rate of ascent and descent was maintained at 300m/min for exposure.
Animal observation and sample collection
Blood was collected from all the groups on respective days in non-pyrogenic and non-endotoxin tubes. Samples were allowed to clot for 2 hours at room temperature before centrifugation at 1000 X g for 10 minutes. Supernatant was collected and stored at -20◦C for storage.
ELISA for detection of Rat CFP by competitive enzyme immunoassay: Rat serum was collected as described above. Standards or samples were added to the precoated wells containing anti-CFP antibody, CFP-HRP conjugate was then added to each well and both were mixed properly. After proper washing, substrate for HRP enzyme was added and the product for enzyme-substrate reaction (Blue-colored complex) was stopped with the stop solution provided in the kit. Intensity of color was measured at 450nm in microplate reader. In the assay, intensity of color is inversely proportional to the CFP concentration because CFP from the samples and conjugate competed for the anti-CFP antibody. Concentration of the samples was calculated using the standard curve plotting O.D. against concentration of standards.
Detection of Rat IgA, C3, C3a, C5, C5a and SERPING1 using sandwich enzyme-linked immune-sorbent assay: Rat serum was collected as described, standard or sample and Biotin-detection antibody working solution was added to each well. Washing was done and HRP-Streptavidin was added to the each well. TMB substrates were added for the blue color product development and reaction was stopped using acidic stop solution to give yellow color. Density of yellow color was proportional to the amount of sample captured in plate. O.D. absorbance was measured at 450 nm in a microplate reader and concentration was calculated by plotting standard curve.
Prism 5 software was used for statistical analysis. Quantitative data was expressed as mean±SEM. One way ANOVA followed by bonferroni post-hoc test was used for comparison between groups. Differences were considered statistically significant at p<0.05.
General conditions of rats
Animals of Control group were found to be free of any infection visible to naked eye, energetic and active. Rats after the HH exposure were found to be less active and more tired.
Change in serum levels
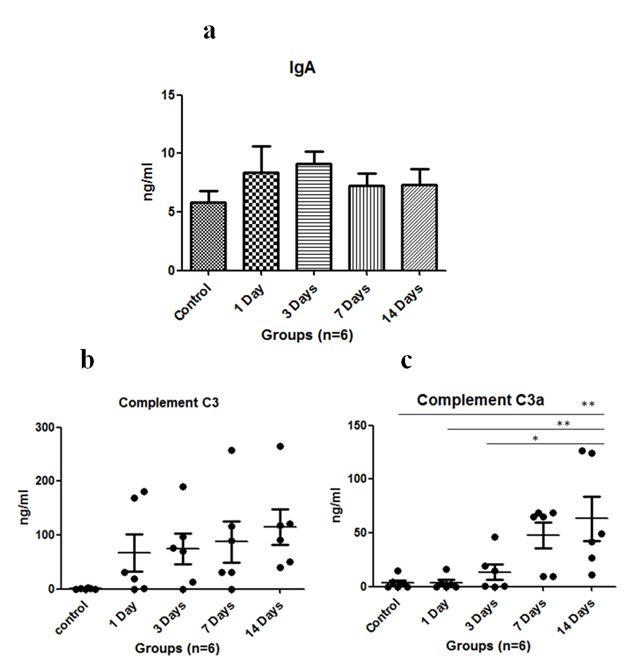
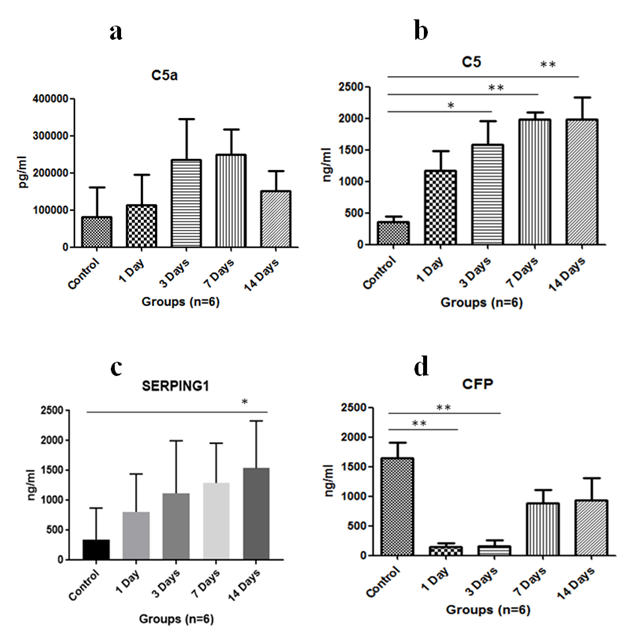
Immunoglobulin such as IgA level was assayed in serum. It was found to increase on exposure to HH on first day and was maximum at 3rd day of exposure, then it started to decrease subsequently (Figure 1A). The complement C3 levels were found to be upregulated with HH and found to be maximum on 14th day (Figure 1B). Anaphylatoxin C3a significantly increased on 14th day of exposure as compared to control where p<0.01 and levels were significantly increased when 1 day exposure was compared to 14 days (p<0.01) and p<0.05 in 3 days versus 14 days (Figure 1C). Anaphylatoxin C5a increased with HH exposure and was maximum at 7th day of exposure (Figure 2A). Whereas, Complement C5 was found to increase upto 14 days of exposure and levels were significant (p<0.05) on 3rd day and p<0.01 on 7th & 14th day when compared to control (Figure 2B). Also, Plasma protease C1 inhibitor (SERPING1) increased with the HH exposure and was found to be maximum at 14th day of exposure with significance of p<0.05 as compared to control (Figure 2C). Complement Factor P significantly decreased (p<0.01) under HH at day 1 and 3 as compared to control. However level of CFP was up-regulated at day 7 and 14 though, it was not found to be significant (Figure 2D).
Vital organs such as lungs, heart and kidneys play an important role in acclimatization at high altitude. Complements and immunoglobulins are the immune system proteins which may affect the kidneys and other vital organs. So far very few studies available on the complement proteins in simulated HH or high altitude. Therefore, in this study we have exposed the SD rats in HH and reported the alterations in complement and IgA level. There are three complement pathways namely classical pathway, alternative pathway and lectin pathway which play an important role in innate and adaptive immunity. Native C3 component is activated by C3 convertase and results in release of C3a, an anaphylatoxin. Another anaphylatoxin C5a is released when C3b component of C5 convertase binds C5 and cleaves it. In our study, we found increased serum C3 as well as C3a levels upto 14 days of HH exposure as compared to control. A study on rats exposed to hypobaric hypoxia at 5000m and 7000m also showed increase in C3 serum component upto 3 days of exposure.7 similarly increased C3a levels were validated with the studies of patients with cancer, burn injury or sepsis, which also dealt with hypoxia.10 In our study, serum C5 and C5a levels were increased with HH exposure. Conditions like sepsis in rats have also shown increased C5 and C5a levels.11 C5 levels have also been found to increase with 1 day of HH exposure at 5000m in a study on rats.7
An interesting study in mice showed that vasodilation can be triggered by increased C5a levels which also correlate with our study as when C5a levels will increase, it will promote vasodilation and hence increase blood flow which help in alleviating the effects of HH.12 CFP or Complement Factor Properdin is only positive regulator for the activation of alternative pathway of complement system.13 The decreased CFP levels showed that the alternate pathway is not activated initially on the exposure to HH. Plasma protease C1 inhibitor or SERPING1 is an inhibitor of complement system which dissociates C1r2s2 from C1q and inhibits the formation of C2 or C4.14 The increased levels showed that the immune system is working to reduce the levels of activated complement proteins back to normal. Serum IgA was found to be increased with HH exposure which is in agreement with the previous studies on stressful conditions & increased immunoglobulin levels.15 In conclusion; a broader knowledge of HH induced alterations in immune system is needed to understand immune changes at high altitude. In our study, HH (7620m) was given to rats for different time durations and analysis of serum complement factors along with sIg A revealed that the immune system of rats is not only affected by the infectious agents or antigens but also by stressful conditions such as HH. Also, complement component activation increases with increasing time of HH exposure. Further studies are needed to understand HH induced immune response changes in humans so as to avoid deleterious effects caused due to HH exposure.
Authors thank Defence Research & Development Organization (DRDO), Ministry of Defence, Government of India, for financial support in the form of project DIP-265. KK thanks DRDO for providing the fellowship in the form of junior and senior research fellowship. We acknowledge Dr. Divya Singh, Ms Sudipta Chanda and Mr Malleswara Rao E for their support in the study.
The authors declare that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.