MOJ
eISSN: 2373-4442


Increasing evidence supports that regulatory T cells (Tregs) within the tumor, tumor draining lymph nodes, ascites and peripheral blood of patients with cancer are associated with poor prognosis. Tregs are important mediators of active immune evasion in cancer. In this review, the potential mechanisms of Treg actions and the roles of Tregs specifically in the tumor microenvironment derived from three types of gynecological cancers, cervical, vulvar and ovarian, are described. The correlations between Tregs and clinical immunotherapeutic study outcomes are discussed. Successful modulation of Tregs would likely have significant impact on the effectiveness of immunotherapeutic treatments in cancer patients.
Keywords: Tregs, cancer, cervical, vulvar, ovarian
Understanding the role of the immune system in the control of cancer growth has been a field of intense investigation over the past decades. The advances in the knowledge of the immune system has led to the view of it as a dual role player in suppressing tumor growth and facilitating tumor progression. The studies in murine models identified tumors as sites of immune tolerance based on the observation that tumor-bearing mice have functional systemic T cell responses with in vitro and in vivo assays despite continued tumor growth.1,2 The concept of T cell suppression was initially established in 1970s. The antigen-specific tolerance could be transferred with antigen-experienced T cells.3 A considerable body of research suggested the existence of in vivo mechanisms of tumor-driven cellular immune suppression.4–7 For example, complete regression of established tumors in a chemically induced fibrosarcoma mouse model, mediated by passively transferred sensitized T cells from immunized donors fail to occur unless the tumors were growing in thymectomized T-cell-deficient recipients.6 Certain CD4 T cell clones selectively down-regulated the induction of cytotoxic anti-immune responses in a human melanoma model.7 In this paper, the functions of regulatory T cells (Tregs), one of the most important immunosuppressive mediators especially in studies of gynecological cancer, are reviewed.
Tregs, as one of the principle cell types responsible for the induction of dominant immune tolerance to tumors, were first identified by Sakaguchi.8 The initial studies by his group demonstrated that elimination of CD25+CD4+ T cells elicited autoimmune disease in a murine model.8 Furthermore, removal of CD4+CD25+ T cells evoked tumor-specific immune responses to syngeneic tumors in vivo and eradicated them in mice.9 The research from Nakayama’s group demonstrated that in vivo administration of anti-CD25 monoclonal antibody caused regression in six of eight murine tumors in syngeneic mice.10 Tregs are divided by lineage into thymic-derived regulatory T cells (tTregs) and peripheral regulatory T cells (pTregs). (Figure 1) tTregs are selected by high-avidity interaction with self-MHC class II-dependent T-cell receptors in the thymus.11,12 pTregs are derived from naïve CD4+ T cells by sub-optimal antigen presentation in the periphery.13 tTregs specifically express the transcription factor forkhead box protein 3 (Foxp3), a “master regulator” of the suppressive lineage while pTregs are generated from Foxp3- precursors.14 Once they are induced, pTregs begin to express Foxp3. It has been shown that expansion of tTregs and de novo generation of pTregs both independently contributed to tumor-specific T cell tolerance in a murine model.15 pTregs comprise two additional Foxp3-subsets interleukin-10 producing Type 1 Tregs (Tr1)16,17 and transforming growth factor-β (TGF- β)-dependent T helper 3 cells (Th3) (Figure 1), which are most commonly associated with oral tolerance.18
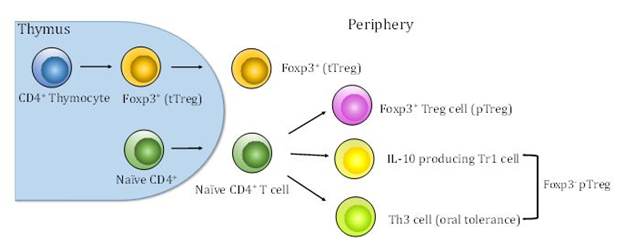
Figure 1 Thymic and peripheral generation of Tregs. tTregs are selected by high-avidity interaction between T cell receptors and self-peptide-MHC class II complexes in the thymus. Peripheral Tregs develop outside the thymus under suboptimal antigen presentation. pTregs are derived from naïve CD4+ T cells. In addition, pTregs comprise two additional subsets Tr1 and Th3.
Accumulating evidence demonstrated an enrichment of CD4+CD25+ Tregs within the tumor mass, peripheral blood, tumor draining lymph nodes or ascites in cancer patients. For example, an increased percentage of CD4+CD25+ Tregs was observed in the non-small cell lung cancer tumor-infiltrating lymphocytes and ovarian cancer tumor-associated lymphocytes.19 Likewise, increased numbers of Tregs was reported in peripheral blood and tumor infiltrating lymphocytes of patients with hepatocellular carcinoma compared to controls including healthy donors and patients with liver disease but without liver malignancies.20 Significantly higher frequency of CD4+CD25+Foxp3+ Tregs in tumor infiltrating lymphocytes was demonstrated in early and advanced stage gastric cancer patients compared to normal gastric mucosa in the same patients.21 Several mechanisms have been proposed for the infiltration and accumulation of Tregs within the tumor microenvironment. One possibility is the recruitment in response to chemokines (Figure 2). It was reported that hypoxic intraperitoneal tumors recruited CD4+CD25+Foxp3+ Tregs through induction of CCL28, known as mucosa-associated epithelial chemokine.22 In addition, ovarian tumor cells and tumor microenvironmental macrophages produced the monocyte derived chemokine CCL22. Monoclonal antibody to CCL22 significantly decreased Treg cells migration into tumors in vivo.23 The second possible mechanism is the preferential Treg expansion. Several lines of evidence indicated that interleukin 2 (IL-2) is essential for Treg development and homeostasis (Figure 2).24–26 The mice deficient in IL-2, or interleukin 2 receptor (IL-2R) were characterized by T cell lymphoproliferation and lethal autoimmunity, which resulted from the absence of functional Tregs.27,28 Ahmadazdeh and Rosenberg reported that the expansion of CD4+CD25+Foxp3+ Tregs was observed following IL-2 treatment in patients with metastatic melanoma and renal cell cancer.29 Likewise, Wei et al.30 demonstrated that IL-2 administration induced the proliferation of Tregs in ovarian cancer patients.30 Another possible mechanism is a de novo differentiation. Tumor derived TGF-β has been shown to induce the Tregs from Foxp3- T cells (Figure 2). The studies from Chen’s group demonstrated that TGF-β and TCR costimulation induced Foxp3 expression in CD4+CD25- naïve responder murine T cells in vitro.31 Moreover, TGF-β converted CD4+CD25+ cells inhibited expansion of antigen-specific naïve CD4+ T cells in vivo in an ovalbumin peptide TCR transgenic adoptive transfer model.31 Furthermore, induction of CD4+CD25+Foxp3+ Tregs in a murine model of pancreas cancer was blocked with anti-TGF-β antibody treatment.32
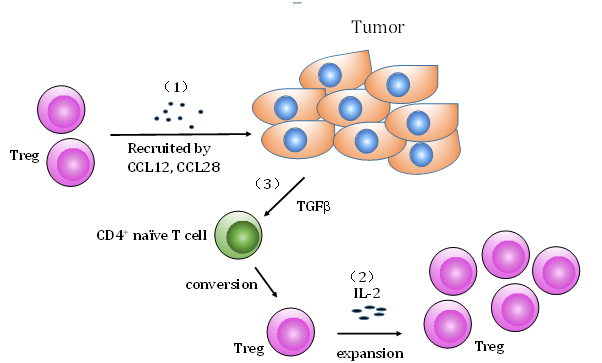
Figure 2Mechanisms of infiltration and accumulation of Tregs in the tumor microenvironment: (1) Tregs are recruited to tumor from the periphery by chemokines e.g. CCL28 or CCL12; (2) Within the tumor microenvironment Tregs can be expanded by IL-2 administration; (3) Tumor derived TGF-β can covert naïve CD4+ T cells into Tregs.
The crucial role of Tregs in tumor immunity is supported in animal models and clinical studies. Depletion of CD4+CD25+ cells with anti-CD25 antibody treatment in a murine model of melanoma resulted in the tumor growth suppression.9 Likewise, Linehan’s group showed that depletion of CD4+CD25+ cells with anti-CD25 antibody alone or in combination with a whole tumor cell vaccine promoted a tumor-specific immune response with enzyme-linked immunospot assay analysis in pancreas cancer-bearing mice.33 They also demonstrated that Treg depletion and vaccination delayed tumor growth and prolonged host survival compared with untreated mice.33 CD8+ T cell-mediated adoptive cell therapy induced the regression of established melanoma in mice, but only when fractionated CD4+CD25- T cells were transferred together with the CD8+ T cells. On the other hand, a co-transfer of unfractionated CD4+T cells (which would still contain Tregs) did not result in tumor regression.34 Administration of multipeptide vaccine (hTERT/survivin) with anti-CD25 monoclonal antibody (mAb), Daclizumab, in patients with metastatic breast cancer patients led to the significant reduction of CD25+Foxp3+ Tregs in peripheral blood.35 Furthermore, effective generation of cytotoxic T lymphocytes specific for TERT and surviving antigens was demonstrated in these breast cancer patients.35 The studies from Dannull et al.36 used the recombinant IL-2 diphtheria toxin conjugate DAB389IL-2, a targeted immunotoxin compound, for depletion of Tregs followed by vaccination with tumor RNA-transfected dendritc cells in metastatic renal cell carcinoma patients. A 7.9-fold median increase of tumor-specific CD8+ T cells was detected in the patients receiving combined treatments compared to a 2.7-fold median increase of tumor-specific CD8+ T cells in the patients receiving vaccination alone.36 Robbins’s team has reported that the levels of peripheral reconstituting CD4+Foxp3+ Tregs in melanoma patients receiving tumor-infiltrating lymphocytes therapy were negatively associated with clinical responses in four clinical trials, which supported the notion that endogenous CD4+ Tregs plays a negative role in cancer immunotherapy.37
The precise suppressive mechanisms of Tregs in the context of tumor immunity are not exclusively defined. In vitro and in vivo studies of Treg cell function indicated that Tregs might use multiple mechanisms, which target various immune cells including the effector T cells, natural killer cells and dendritic cells (Figure 3).

Figure 3 Mechanisms of Tregs-mediated immunosuppression. Tregs promote tumor progression by (1) inhibition of effector T cell proliferation, lysis of effector T cells through release of granzyme B and perforin, or conversion of CD4+ T cells into Tregs; (2) interactions with DCs through downregulation of CD80/CD86 on DCs or upregulation of indoleamine 2,3-dioxygenase IDO in DCs; (3) inhibition of NK cell function through downregulation of NKG2D on NK cells or direct inhibition of NK proliferation and cytotoxicity.
Influence of tregs on effector T cells
Many groups have demonstrated that CD4+CD25+ T cells potently suppress proliferation of other CD4+ and CD8+ T cells when Tregs and responder cells were co-cultured and stimulated with specific antigen or anti-CD3 mAb.38,39 Some studies have shown that Tregs can kill effector T cells directly in culture through the release of granzyme B and perforin.40–42 In addition, Tregs can alter the differentiation of other T cells.43–45 Jonuleit et al.43 found that coculture of human CD4+C25+ Tregs and CD4+C25- T cells not only suppressed the proliferation of conventional CD4+ T cells but also induced suppressive activity in these CD4+ T cells, resulting in the development of additional CD4+ suppressor T cells in vitro.
Modification of antigen presenting cells by treg Cells
Cederbom et al.46 has reported that CD4+CD25+ Tregs down-regulated the expression of the co-stimulatory molecules CD80 and CD86 on dendritic cells.46 In addition, several studies showed that Treg cells can stimulate antigen presenting cells to upregulate the activity of indoleamine 2,3-dioxygenase (IDO), which is a potent immunosuppressive enzyme that promotes peripheral immune tolerance by inhibiting T-cell activation and proliferation.47–49 On the other hand, Chung and colleagues found that mature human monocyte-derived dendritic cells expanded Tregs by an IDO-dependent mechanism.50 These observations indicated that there is a two-way communication between Tregs and dendritic cells.
Inhibition of natural killer (nK) cell function by treg cells
Ghiringhello et al.51 demonstrated that human Tregs directly inhibited NK cell functions and down-regulated NKG2D receptors on the NK cell surface.51 They also provided the evidence that human NK cell-mediated tumor recognition could be restored by depletion of Treg cells from tumor infiltrating lymphocytes.51 It was reported that in vitro NK cell proliferation and cytotoxicity towards tumor targets were inhibited by Tregs.52 Barao and colleagues demonstrated that NK cell-mediated bone marrow cell rejection was significantly augmented with prior Treg depletion of the recipient mice.53
The contributions of Tregs to the tolerogenic tumor microenvironment in the human gynecological cancer especially in the context of cervical cancer, vulvar cancer, and ovarian cancer are evaluated in this review. Such acquired body of knowledge would be essential for the development of effective immunotherapeutic strategies against human gynecological cancer.
Tregs in human cervical cancer
Cervical cancer is the third most common cancer among women worldwide.54 An estimated 12,820 case of cervical cancer will be diagnosed and an estimated 4,210 deaths will occur in the US during 2017.55 Persistent infection with high-risk human papillomavirus (HPV) including HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, 82 has been shown to be an important risk factor for the development of cervical cancer and its precursor lesions termed cervical intraepithelial neoplasia (CIN).56 Of the high-risk HPV types, HPV type 16 is the most common types in malignant lesions, responsible for over 50% of invasive cervical cancer.57 Five-year survival rates for the patients diagnosed with localized, regional and distant-stage disease of cervical cancer are 91%, 57%, 17% respectively.55 Various investigations have indicated that development of HPV-induced cervical cancer is associated with the failure to induce the HPV specific type1 T-helper and cytotoxic T lymphocyte responses.58–61 Furthermore, several studies have identified the immunosuppressive microenvironment established during HPV associated cervical carcinogenesis.62–66 Multiple types of immunosuppressive cells including tumor-associated macrophage (TAM), regulatory dendritic cells, Tregs, are recruited and activated at the tumor sites.64‒67 Of note, accumulating data demonstrated that increased levels of Tregs were present at the cervical tumor site and in the lymph nodes and peripheral blood of patients with CIN or cervical cancer.65–70 Characterization of Tregs using antibodies to Foxp3 was performed in six groups including HPV-positive cervicitis, HPV-negative cervicitis, CIN III, CIN II, CIN I, and squamous cell carcinoma (SCC).68 Foxp3 positive cells were detected in all invasive tumors of SCC (30 cases) compared to only 12 of 30 cases in the CIN III group. They were detected in 5 of 11 cases in the patients of CIN Il group, 4 of 10 cases in the CIN I group, 1 of 30 cases in the HPV positive cervicitis patients, and none of 7 HPV negative cervicitis patients. 68 Moreover, the ratio of CD4+ T-cells to Foxp3 positive cells and that of CD8+ T-cells to Foxp3 positive cells were significantly reduced in the SCC group (11±8 and 11±8 respectively) compared to the CIN III group (43±29 and 47±25 respectively) with immunostaining analysis.68 Van der Burg and colleagues isolated HPV-specific CD4+ Tregs from lymph node biopsies of cervical cancer patients, which has been shown to suppress proliferation and cytokine interferon gamma (IFN-γ) and IL-2 production by responder T cells.71 In addition, Fattorossi’s group examined the immune cell populations in metastatic tumor draining lymph nodes (mTDLN) and metastatic free tumor draining lymph nodes (mfTDLN), and discovered that CD4+Foxp3+ Tregs were more significantly abundant in mTDLN than mfTDLN.62 Moreover, Jordanova and colleagues showed that a high number of intraepithelial Tregs and a low CD8+ T-cell/Treg ratio were associated with worse survival in 115 cases of cervical cancer.72 CD8+ T-cell/Treg ratio was demonstrated to be the only single variable independent prognostic factor by multivariate statistical analysis in this study.72 Taken all together, cumulative evidence indicated that Treg may suppress the immune control of cervical neoplasia. Daemen’s group showed that in vitro depletion of CD25+ Tregs from HPV16-positive cervical cancer patients led to the increased IFN-γ T cell responses against HPV16 E6 and E7 peptides.73
The studies from Cichon’s research team demonstrated that inactivation of Tregs by agonistic anti-glucocorticoid-induced tumor necrosis factor receptor family-related protein (GITR) antibodies induced strong intra-tumoral invasion of CD8+ T cells and complete tumor eradication in 70% of treated animals in a murine model of cervical cancer.70 Several therapeutic vaccines applying peptide or protein-based, vector-based, and cell-based strategies have been developed to treat patients with premalignant cervical and cervical cancers.74–83A Phase I clinical trial with a human HPV therapeutic vaccine PepCan, which consists of four current good-manufacturing production-grade peptides covering the HPV16 E6 protein and Candida skin test reagent as a novel adjuvant, was evaluated in 34 women with cervical intraepithelial neoplasia CIN2/3 in our group. The observed overall histological response was 45% (14 of 31 patients who completed the study). Noticeably, pre-vaccination regulatory T cell levels were significantly lower in histological responders compared to non-responders (p = 0.03).82,83 Ferrara and colleagues reported that dendritic cell-based tumor vaccines pulsed with recombinant HPV16 E7 or HPV18 E7 oncoprotein were administered to 15 stage IV cervical cancer patients.76 Induction of IFN-γ secreting T-cell response was found in 3 out of 11 evaluated patients. However, no objective clinical response was observed in this study.76 Patients with HPV16-positive advanced or recurrent cervical cancer were vaccinated with an HPV16 synthetic long peptide vaccine consisting of the HPV16 E6 and E7 oncoproteins in Montanide adjuvant.79 The 9 out of the 11 vaccinated patients have shown the vaccine-induced HPV16-specific IFN-γ associated immune responses.79 However, no tumor regression was observed among the vaccinated patient.79 Also, compared to a cohort group of non-vaccinated patients, the median survival time among the vaccinated patients was not significantly different (8.5±9.4 months in vaccinated group vs. 11.0±7.7 months in matched cohort group, p = 0.59).79 The same group also has shown that HPV16 synthetic long peptide vaccine induced HPV16-specific CD4 and CD8 T-cell responses in all six postoperative HPV16 positive cervical cancer patients.78
Expansion of CD4+CD25+Foxp3- type 1 cytokine IFN-γ producing T cells as well as CD4+CD25+Foxp3+ T-cell population was observed in vaccinated patients.78 The two patients who displayed the local recurrence after vaccination had mounted the similar magnitude of percentage of HPV16-specific CD4+CD25+Foxp3- T-cell subset and CD4+CD25+Foxp3+ T-cell subset. However, the other evaluable three patients who had no sign of recurrence during the time of follow-up had 3.9- to 11.4 -fold higher of percentage of HPV16-specific CD4+CD25+Foxp3- subset than that of CD4+CD25+Foxp3+ T-cell subset.78 In the study of Stevanovic et al.,84 nine patients with metastatic cervical cancer followed by chemotherapy previously were treated with tumor-infiltrating T cells selected for reactivity against HPV E6 and E7 proteins. Two of nine patients attained complete and one patient received partial response.84 This encouraging report indicated that properly activated T cells in adoptive T-cell therapy can produce tumor regression in patients with advanced cervical tumor. It is also noteworthy that combination of chemotherapy with adoptive T-cell therapy in this clinical trial might have a synergistic effect since several chemotherapy reagents have been shown to modulate the tumor microenvironment such as by eliminating Tregs or inducing the macrophage chemoattractant protein-1.85–87 In the past, various immunotherapy clinical trials showed little clinical benefits especially in advanced cervical cancer patients. More recently, encouraging results are emerging using strategies to overcome immunosuppressive mechanisms to improve applicability of immunotherapy.
Tregs in vulvar cancer
Similar to cervical cancer, persistent infection with high risk types HPV including HPV16 are associated with vulvar cancers, which constitute 5.6% of all gynecologic cancers.88,89 The incidence of vulvar intraepithelial neoplasia (VIN), a premalignant condition, which is increasing with 60-75% occurring in women under age of 50.90–92 An estimated 6,020 of vulvar cancer cases will be diagnosed and an estimated 1,150 deaths will occur in the US during 2017.55 The risk of progression from VIN to invasive cancer is from 3.8% to 9%.93,94 Recurrence is a particularly problematic feature of vulvar cancer cases.95 Van Esch et al.96 reported that the usual-type vulvar intraepithelial neoplasia (uVIN) lesions, the most common VIN type lesions, were infiltrated by high numbers of Tregs.96,97 They also found that a low CD8+TIM3+ T cells infiltration combined with higher infiltration of Tregs was negatively associated with the recurrence in uVIN.96 Importantly, compared to that in the uVIN tissues, the ratio of Treg/ CD8+TIM3+ T-cell in vulvar carcinoma tissues progressively increased.96 Vaccination of a synthetic long-peptide against the HPV-16 oncoproteins E6 and E7 in women with HPV-16 positive, grade 3 vulvar intraepithelial neoplasia displayed a 47% (9 of 19 patients) complete regression (CR).98,99 Compared to non- or partial responders, patients with CR mounted higher ratio of HPV16-specific effector T cells to HPV16-specific CD4+CD25+Foxp3+ Tregs. A combination of imiquimod, an immune response modifier, and HPV therapeutic vaccine comprising a HPV16 E6E7L2 fusion protein was given in 19 women with VIN grads 2 and 3 in a phase II clinical trial conducted by Daayana et al.100 The effects of Imiquimod are mediated though agonistic activity towards toll-like receptors (TLR) 7 and 8, leading to activation of antigen-presenting cells.101 Complete regression of VIN was observed in 32% (6 out of 19 patients) in this study. Significantly increased local infiltration of CD8 and CD4 T cells was observed in responders, but an increased density of Tregs was identified in non-responders.100 The studies from these promising trials suggested that in the setting of premalignant disease, therapeutic strategy could potentially reach clinical efficiency through Treg modulation.
Tregs in ovarian cancer
Ovarian cancer accounts for 5% death among the women, causing more death than any other gynecological cancer. An estimated 22,440 ovarian cancer cases will be diagnosed and an estimated 14,080 deaths will occur in the US during 2017.55 The 5-year relative survival rate for ovarian cancer is about 46%.55 Ovarian cancer is usually diagnosed in advanced stages due to the lack of obvious symptoms in the patients and effective screening methods. Current treatment includes debulking and chemotherapy with paclitaxel and platinum agents. In spite of the significant advances in surgery and chemotherapy, recurrence still occurs in about 70% of the patients who become refractory to further chemotherapies.102,103 It has been shown that clinical outcome and five-year survival rate in patients are positively associated with the number of CD3+ tumor-infiltrating cytotoxic T lymphocytes,104,105 which suggested that host immunity plays an important role in the course of ovarian cancer. Instead of being targeted for immune destruction, ovarian cancer has the ability to escape the immune system by creating a highly suppressive environment in the peritoneal cavity. Tregs, tolerance-inducing plasmacytoid dendritic cells (PDCs), B7-H4+ macrophage, immune-suppressive cytokines such as interleukin-10 (IL-10) and TGF-β are present in the ovarian cancer environment,23,106–109 which indicated that multiple cellular and molecular components created the immune suppressive network in ovarian cancer. High numbers of PDCs were found in malignant ascites of ovarian cancer patients.107 Tumor-associated PDCs induced angiogenesis in vivo through production of tumor necrosis factor α and interleukin 8. Kryczek et al.108 reported that ovarian tumor macrophages expressed high level of B7-H4 molecules, a negative regulator of T cell responses in vitro by inhibiting T cell proliferation, cell cycle progression, and cytokine production.108
They also demonstrated that tumor environmental interleukin 6 (IL-6) and IL-10 induced macrophage B7-H4 expression.108 Curiel et al.107 observed significant accumulation of CD4+CD25+Foxp3+ Tregs in malignant ascites and tumor tissues from 104 individuals with untreated ovarian cancer patients whereas CD4+CD25+Foxp3+ Tregs were undetectable in normal ovarian tissues without cancer.23 They demonstrated that accumulation of tumor Tregs predicts poor survival in individuals with ovarian cancer.23 In addition, Wolf and colleagues showed that high Foxp3 expression from 99 ovarian cancer patients was associated with poor prognosis in terms of overall survival (p = 0.0034) and progression-free survival (p = 0.0041).110 Studies from Sata et al.111 reported that CD8+ tumor infiltrating lymphocytes (TILs) and a high CD8+ TILs/Tregs ratio were associated with favorable prognosis in ovarian cancer.111 Curiel et al.107 reported that tumor cells and tumor macrophages produced the chemokine CCL22, which mediated Tregs trafficking to tumor.23 Interestingly, they also found that tumor Tregs triggered macrophage to produce high levels of IL-10, which was responsible for B7-H4 expression.109–112 The data from these studies implied that immunosuppressive network mechanisms might be established in the ovarian cancer. Successful ovarian cancer vaccine therapy might require the effective blockade of multiple immune-tolerance mechanisms. Several immunotherapy approaches have been used in ovarian cancer including therapeutic vaccines, monoclonal antibodies, checkpoint inhibitors and adoptive T cell transfer.113–118 Most of these therapies are still in early-phase testing. Odunsi et al.114 conducted a phase I clinical trial immunization with an NY-ESO-2 peptide in 18 ovarian patients.114 NY-ESO-1 is one of the most spontaneously immunogenic tumor antigens in testis and ovary.119 They have demonstrated that tumor-reactive CD4+CD8+ T cell responses were detected in ovarian cancer patients. However, clinical benefit afforded by vaccination has been marginal.114 A phase II trial using anti-PD-1 monoclonal antibody, Nivolumab, in 20 patients with platinum-resistant ovarian cancer showed encouraging results.116 Programmed cell death-1 (PD-1), an immune checkpoint receptor expressed by T cells, binds to two PD-1 ligands PD-L1 and PD-L2, and suppresses antigen-specific cancer immune reaction.120,121 PD-L1 expression in tumor cells is associated with poor prognosis in ovarian cancer and PD-L1 enables immune evasion during peritoneal dissemination by inhibition of cytotoxic T-lymphocyte function.122 The overall response in this trial was 15% and the disease control rate was 45%.116
Clinical efficacy of immunotherapy may be enhanced by attempting to reduce immunosuppressive mediators such as Tregs. Certain chemotherapy regimens such as low dose of cyclophosphamide has been shown to reduce the number and function of Tregs that resulted in the increased immune response.123–125 For example, low-dose (300 mg/m2) cyclophosphamide treatment has yielded enhanced immunological and clinical responses when delivered in conjunction with hapten-modified melanoma vaccines and with THERATOPE STn-KLH vaccination in breast cancer patients.126,127 A combinational approach of a new dendritic cell vaccine for recurrent ovarian cancer in combination with antiangiogeneis therapy and metronomic cyclophosphamide was reported and awaited for future evaluation.87
During the past decade, remarkable progress has been made in understanding the interaction between the immune system and cancer. It is now appreciated that the immunological response against cancer is a critical balance between immune-activating and immune-suppressing mechanisms. The current wealth of information in the studies of tumor microenvironment in patients from cervical cancer, vulvar cancer and ovarian cancer indicated that Tregs are significant contributors to tumor-associated immune suppression. The knowledges in the functional studies of Tregs in each specific gynecological cancer type may provide new treatment strategies to effectively manipulate Tregs through methods such as depletion, blocking trafficking, and alleviating suppressive mechanism. It would be of interest to examine cancer therapy-induced changes on elimination or activation of Tregs in tissues and peripheral circulation of cancer patients. A combinational cancer vaccine combined with Tregs modulation would be a promising approach to attain an effective antitumor responses.
This study was funded by National Institutes of Health grants RO1 CA143130 and P50 CA136393.
Mayumi Nakagawa is named as one of the inventors of PepCan in patents and patent applications describing it. Martin Cannon and UAMS have a financial interest through licensed patents in aspects of the technology discussed in this publication. These financial interests have been reviewed and approved in accordance with the UAMS conflict of interest policies.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.