MOJ
eISSN: 2575-9094


Research Article Volume 1 Issue 1
1Department of Pharmaceutical Sciences, Larkin Health Sciences Institute, USA
2School of Pharmacy, South University, Savannah, USA
3Department of Pharmaceutical Sciences, Mercer University, USA
Correspondence: Mohammad Nasir Uddin, Department of Pharmaceutical Sciences, College of Pharmacy, Larkin Health Sciences Institute, Miami, Florida 33169, USA, Tel 305-760-7472
Received: September 08, 2016 | Published: September 20, 2016
Citation: Uddin MN, Cotta KI, Dsouza MJ. Stability determination and evaluation of gamma-irradiated nuclear factor-κB antisense microsphere. MOJ Drug Des Develop Ther. 2016;1(1):1?6 DOI: 10.15406/mojddt.2016.01.00001
Gamma irradiation is the most common sterilization process used in the pharmaceutical industry. Here, we have determined the stability and integrity of a NF-kB antisense after applying gamma radiation. A radiation of 25kGy was applied to the microencapsulated antisense. The micro particle was characterized by SEM, Zeta potential, and HPLC before and after gamma-irradiation for evaluation. An in vitro study was conducted to determine the biological functionality. The size of the particle before and after radiation was similar. The zeta potential value was negative in both cases. The HPLC analysis showed that the antisense was intact after gamma irradiation. In vitro study showed the gamma radiated antisense blocks TNF-µ and IL-1b cytokines. The microsphere was unchanged during the gamma irradiation in terms of size, shape, surface morphology and surface charge. No degradation of the antisense drug during the radiation process was observed and is biologically active.
Keywords: gamma radiation, microspheres, albumin, NF-kB, cytokine, antisense
In the pharmaceutical industry, all pharmaceutical components, including active pharmaceutical ingredients (API), excipients, final drug products, laboratory equipment, and medical devices, need to go through a sterilization process. During sterilization process, microorganisms, such as fungi, bacteria, viruses and spores, are killed or eliminated. Various sterilization methods are used depending on the purpose of the sterilization and the type of the material that will be sterilized. These methods include: dry heat sterilization, pressured vapor sterilization, ethylene oxide (EtO) sterilization, formaldehyde sterilization, gas plasma (H2O2) sterilization, per-acetic acid sterilization, e-beam sterilization and gamma sterilization.1 Depending on their different mechanism of action, these sterilization methods affect the pharmaceutical formulations in different ways. Thus, a suitable sterilization process should be selected based on the compatibility of the item that needs to be sterilized to avoid any damage. Gamma sterilization is commonly used for pharmaceutical drugs, products and equipment. Gamma-irradiation is a physical means of decontamination, which destroys bacteria by breaking down bacterial DNA, consequently inhibiting bacterial growth.2 Gamma rays pass through products and equipment, disrupting the pathogens that cause contamination. These photon-induced changes at the molecular level cause the death of contaminating organisms or render such organisms incapable of reproduction. Gamma-irradiation is a terminal sterile method that has some advantages over the other sterilization processes. For example gamma-irradiation can be applied on drugs in their finished product or final container. Gamma-irradiation is also capable of delivering rays over a period of time ranging from minutes to hours depending on the thickness and the volume of the product.3In addition, gamma sterilization has the high penetration power and possesses a unique isothermal character that allows for a suitable treatment of heat-sensitive materials with minimum temperature raise.4 Moreover, gamma-irradiation assures homogeneous sterilization and can be used efficiently for packaged products avoiding further risk of microbe contamination. The standard dose of gamma-irradiation is 25kGy considering the number and resistance of naturally occurring contaminating microorganisms in raw materials.5Although very precise and accurate in applying doses, gamma-irradiation sterilization has some drawbacks. The energy transfer by the radiation can induce fragmentation of covalent bonds and production of free radicals that, in turn, are responsible for the majority of the damage that occurs to irradiated materials. Free radicals are chemical compounds that possess an unpaired electron in the outer shell of the molecule and due to this unpaired electron, these species are paramagnetic.6 Measurements of free radicals in food and biological systems by electron spin resonance spectroscopy (ESR) are widely used in the fields of food irradiation, lipid oxidation, antioxidants, and food processing.7–8 In addition, gamma-irradiation can cause alteration of the physico-chemical properties of the material, decrease of the amount of active ingredient by partial decomposition, and/or create molecular fragments that may result in a toxicological hazard.9Moreover gamma-irradiation can cause any substantial modifications to a polymer.10–11 Consequently, for pharmaceuticals that contain polymers as formulation components, ionizing irradiation could generate degradations that adversely affect the behavior of the polymer. Therefore, it is obligatory to verify the integrity of a formulation composed of polymers that goes through the process of gamma-irradiation for sterilization purpose. In this study, we have determined the stability of a microencapsulated antisense after sterilization using gamma-irradiation. Encapsulation of drug molecules with polymers is an important aspect of drug delivery that facilitates effective therapy by targeting the drug entities as well as protecting the molecules from adverse conditions, such as harsh acidic environment or enzymatic degradation before reaching the target.12 Antisense oligonucleotides are short single stranded DNA or RNA molecules (15-25 nucleotides) which are effective blocking agents for protein expression with high sequence specificity.13 Antisense drugs are less stable in blood serum, when delivered orally, because they are prone to enzymatic degradation in the body. Hence, enhanced stability of the antisense drug after oral administration is desirable for its therapeutic use.14 When antisense is microencapsulated with a biodegradable polymer, it has to be sterilized before administration. Gamma-irradiation is commonly used for antisense micro particle sterilization, but the radiation may have adverse effect on the particles. Gamma-irradiation can cause not only the degradation of the antisense, but also the polymer that encapsulates the antisense. Therefore, it is very crucial to determine the stability and integrity of the polymer/drug after gamma-irradiation. The goal of the present study is to determine the stability, integrity and biological activity of the microencapsulated antisense after radiation sterility.
Materials and chemicals
Antisense oligonucleotides specific to the rat and mouse mRNA sequence of the NF-kB p65 subunit were obtained from TriLink Bio Technologies (San Diego, CA). The sequence of the NF-kB p65 antisense oligonucleotides was provided by AVI Biopharma (Corvallis, Oregon) and was as follows: 5′-GGA AAC ACA TCCTCC ATG-3′. The oligonucleotides were purified using anion exchange HPLC and were packaged as a lyophilized solid. Bovine serum albumin (BSA; Fraction V, DNAase, RNAase, and protease-free) was obtained from Fisher Scientific (Norcross, GA). The albumin was used as the oligonucleotide encapsulation matrix. Glutaraldehyde (25% in water), used as the cross-linking agent, was obtained from Fisher Scientific (Norcross, GA). Oligreen assay kit and buffered phenol were procured from Invitrogen Corporation (Carlsbad, CA). All chemicals used in the study were of analytical grade and purchased from Fisher Scientific (Pittsburg, PA). Protease (DNase-free and RNase-free) was procured from Roche (Nutley, NJ). All the tubes, tips and water were autoclaved before use.
Equipment
A Buchi Mini Spray Dryer used in the spray drying process to produce micro particle was obtained from Buchi Corporation (Newcastle, DE). The Horiba LA920 laser scattering particle size distribution analyzer that was used to determine microsphere particle size was obtained from Horiba Instruments Incorporated (Irvine, CA). The Malvern Zetasizer Nano ZS that was used for zeta-potential analysis was obtained from Malvern Instruments (Worcs, UK). The JEOL JSM-5800L scanning electron microscope used for surface morphology analysis was obtained from JEOL USA (Peabody, MA). ELIZA kit for in vitro whole blood study was obtained from Boster (Pleasanton, CA).
Escherichia coli culture
Gram-negative bacteria E. coli (serotype 086:K61) was obtained from ATCC (Rockville, MD) and grown in a trypticase-soy broth medium by incubation at 37°C in presence of 5% CO2. The bacterium E. coli was concentrated by centrifugation and the final volume was reduced to an amount which can give an optical density (OD) of 650 nm spectrophotometrically. In this range each milliliter of bacteria colony contained approximately 1010 colony-forming units (CFU) of E. coli.
Preparation of microspheres
NF-kB antisense drug loaded microspheres (10% drug loading) were prepared by dissolving the particles in deionized water. BSA solution of 2% w/v in deionized water was pre-crosslinked with glutaraldehyde.15 The 18-mer oligonucleotide (TriLink BioTechnologies) weighted with respect to BSA were then added, and the solutions were spray-dried using a Buchi 191 mini spray dryer. The inlet and outlet temperatures were 110oC and 80oC, respectively. The micro particles were collected, weighed and used for further characterization.
Characterization of microspheres
The size of the microspheres and the size distribution were measured using a Horiba LA920 laser scattering particle size distribution analyzer with a (1mg/ml) suspension of microspheres in Milli-Q ultra-pure water. The surface morphology of the particles was determined by scanning electron microscopy using a JEOL JSM-5800L scanning microscope (Peabody, MA) following gold-palladium splutter coating. The zeta potential of the particles was measured using a Malvern Zetasizer Nano ZS (Worcs, UK) with a (500μg/ml) suspension of antisense microspheres in Milli-Q ultra-pure water.
Gamma-irradiation
Irradiations of the microspheres were carried out at room temperature with a 60Co panoramic irradiator IGS-3 at a dose of 25 kGy. The dose of 25 kGy was selected as it is the recommended doses for sterilization treatment of drugs at the industry level.5 Non-irradiated samples were kept as reference.
Stability study of gamma-irradiated microspheres
A suitable saturated salt solution was prepared to create the storage conditions for the stability test. Sufficient amount of analytic reagent grade sodium chloride salt was placed in a vessel was added with 1100 mL of water to prepare a thick slurry. The vessel was then placed in a desiccator which was placed in an oven at 42ºC. Accelerated storage conditions were maintained at 42°C and 75% use full form for 6 months. Accelerated condition is a condition designed to increase the rate of chemical degradation or physical change of a drug substance or drug product by using exaggerated storage conditions as part of the formal stability studies. Data from these studies, in addition to long-term stability studies, can be used to assess long-term chemical effects at non-accelerated conditions and to evaluate the effect of short term excursions outside the label storage conditions. The stability of the microsphere was determined six months after applying the gamma-irradiation.
Extraction of antisense from microsphere
Blank microspheres, antisense encapsulated microspheres, and gamma-irradiated microspheres with antisense to NF-kB were processed for quantification according to the following protocol. First, the blank BSA microspheres, encapsulated antisense to NF-kB, and gamma-irradiated encapsulated antisense to NF-kB were weighed (6.1, 4.9 and 5.1 mg, respectively) in 1.5 ml microfuge tubes and 200 µl of buffer 1 (50 mM, Tris-HCl, 10 mM EDTA, pH 8.0) was added to each tube. The tubes were placed in a 37ºC water bath and incubated for 4 h with vortexing at regular intervals. Then 200 µl of buffer 2 (200 mM NaOH, 1% SDS) was added to each tube and vortexed. After 30 min of addition of buffer, protease was added to each solution for digestion to achieve a final concentration of 100 mg/ml. The solutions were then placed in a 37ºC water bath for 12 h (overnight). At the end of the incubation period, the solutions were centrifuged for 20 min at 14,000 rpm and 250 µl of the clear upper layer from each vial were collected. Then 100 µl of supernatant was placed in another micro-centrifuge tube and 100 µl of buffered phenol was added to each vial. The contents were mixed several times. These solutions were then centrifuged at 14,000 rpm for 10 min and the clear supernatants were collected. The aqueous supernatant was transferred to a clean Eppendorf tube. Equal volumes of chloroform/isoamyl alcohol (IAA) (24: 1, v/v) were added and mixed. Centrifugation was performed as above and the supernatant was used for the oligreen fluorescence assay.
HPLC analysis of antisense from microspheres
Gradient HPLC analysis, using a protocol developed for NF-kB antisense, was performed to determine if any structural modifications or degradation of antisense occurred due to gamma-irradiation of microspheres.12 HPLC was conducted with antisense isolated from NF-kB microspheres and gamma-irradiated NF-kB microspheres. Pure NF-kB antisense was used as standard. The HPLC parameters used are provided in Table 1. All HPLC grade solvents (mobile phase, water and methanol) were filtered using 0.45 mm Millipore filters and were degassed by sonication. The acetonitrile and 50 mmol sodium phosphate buffer (pH 7.4) were used as the mobile phase. The sodium phosphate buffer was prepared by mixing 11 mmol monosodium phosphate monohydrate and 38 mmol of anhydrous disodium phosphate. Detection of the antisense-oligonucleotide in the eluants was done by using UV detection at 254 nm. The HPLC gradient profile is depicted on Table 2.
Column |
C-18 |
Temperature (°C) |
40 |
Injection volume (µl) |
20 |
Flow rate (mL/min) |
1 |
Mobile phase |
Na-phosphate buffer : Acetonitrile (B:C) |
Elution |
Gradient |
Retention time (min) |
14.5 |
Table 1 HPLC parameters used to analyze NF-kB antisense microsphere before and after gamma-irradiation.
Time (Min) |
Flow (ml/min) |
% of Buffer |
% of Acetonitrile |
Curve |
Initial |
1 |
95 |
5 |
NA |
3 |
1 |
95 |
5 |
1 |
4 |
1 |
80 |
20 |
6 |
15 |
1 |
80 |
20 |
1 |
16 |
1 |
95 |
5 |
6 |
25 |
1 |
95 |
5 |
1 |
Table 2 HPLC gradient profile of NF-kB antisense microsphere.
In vitro whole blood study
To investigate the biological activity of the antisense microsphere, the in vitro whole blood study was conducted. In this study, the effectiveness of microencapsulation of antisense to NF-kB antisense microspheres was compared before and after gamma radiation to block the TNF-α and IL-1b cytokines. Elaborated description of the method of the experiment has been published before.16 Briefly, the whole blood (35 ml) was drawn from 6 normal human volunteers who were healthy and taking no medications. The blood was separated into aliquots and placed into 1-ml wells. E. coli endotoxin (10 µg) was added to each sample, and samples were incubated. Samples were analyzed by ELISA after 4 h incubation for TNF-α and 24 h for IL-1b.The following groups were studied: NF-kB microsphere group before radiation, gamma-irradiated NF-kB Microsphere group, saline group and NF-kB solution group. All the NF-kB groups contain 200 µg/mL concentration of antisense. The saline group was the control group. Each group contained equal amount of endotoxin (10 µg). The blocking activity was determined by measuring the TNF-α and IL-1b level using ELISA. The result of this study is shown in the Figure 1.
The antisense oligonucleotide containing microspheres were prepared using biodegradable albumin (BSA) polymer in a one-step spray drying process. In the spray drying process, the albumin polymer was crosslinked using glutaraldehyde to construct the matrix where the antisense drug was entrapped. Glutaraldehyde acts as a coupling agent by linking the albumins via amide bond formation. By controlling the inlet temperature and pressure, and by maintaining required time for the particles in the spray dryer allows for the production of the desired micron size particle. The frequency and size distribution data of the microspheres indicated that 90% of them were in the range of 2-8.6 μm. The mean size was 4.7 µm with a polydispersity index of 0.435. The polydispersity index indicated that relative size uniformity is between 4 and 7 µm. Size distribution data analysis indicated about 20% of the microspheres were less than 3 μm in size (Figure 2). Size determination of the micro particles is of importance as the size may influence the syringe insert-ability and inject-ability properties of a parenteral suspension if the particles are injected via parental routes. Size is closely related to the particle characteristics of the parenteral suspension. Generally if the size is less than 10 µm, it minimizes the pain and irritation and is more patient compliant. The rate of reconstitution from a drug powder to form aqueous solution/suspension is influenced by the particle size as well.17 Also the rate of release of drug depends on the size of the particles, generally smaller the size higher is the release rate.18 Moreover, the particle size is very important for drug targeting. The surface morphology of the particles was also determined. The surface morphology of the NF-kB antisense micro particles displayed a spherical morphology (Figure 3). The shape of the particles may affect degradation, transport, targeting, internalization and possibly other areas of drug delivery. Although the effect of shape on biological interactions is not as clear as physical behavior such as degradation, flow rate, there are significant observations of dependence of phagocytosis on shape, which demonstrate the complexity of cell-particle interactions and reveal the ability of cells to sense, identify and respond to particle shape.19 Zeta potential was also determined and found to be -15 mV (Figure 4).

Figure 3 Scanning electron microscope picture of NF-kB antisense oligonucleotide containing microsphere before gamma-irradiation.
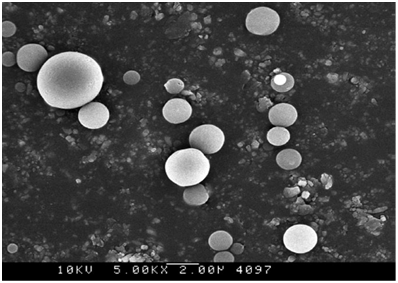
Figure 4 Scanning electron microscope picture of NF-kB antisense oligonucleotide containing microsphere after gamma-irradiation.
Micro particles were irradiated with 25 kGy gamma-irradiation, a standard radiation process. The particles were then kept in an appropriate condition for stability testing for six months. The standard stability conditions were maintained at 42°C and 75% RH for 6 months. Particle characterization was then repeated six months after applying gamma-irradiation for stability determination. The particle shape remained spherical following gamma-irradiation (Figure 3) and the size was 1 to 8 µm, and the zeta potential value was -42 mv (Figure 5). The spherical shapes of the micro particles assist the particles in uptake and diffusion. The surface morphology would be significantly different if the particle was affected by radiation as it would cause fragment formation or distortion of the particle due to breakage of the polymer matrix. Particle size is also important in terms of particle uptake. Our data clearly shows that the particle size remained within the same range [Figure 3], which clearly indicates that the particles remain unchanged following gamma-irradiation. For micro particle formulations, 3-5 micron size range has been shown to be ideal for oral delivery.20 The suitable size range for phagocytosis is 1-7µm.21,22 Thus, the NF-kB antisense particle will be similarly functional in terms of size after gamma-irradiation. The zeta potential of the particles after gamma-irradiation was -40 mV (Figure 5). The zeta potential value is important as positive or negative surface charge will influence the interaction of the particle with cell surface and/or particle uptake. Having a surface charge of zero would cause aggregation of the particle. In this study the zeta potential of the surface of antisense microsphere changed from -15 mV to -40 mV after gamma radiation. Significant zeta potential change may attribute to the pH change, presence of excess ionic group.23 Also even though the zeta potential is given by a value it in fact it represents a range. In this case the change is not significant as the both are negative values and the range is also very close. The negative zeta potential value of the surface is due to the anionic charge of the macromolecular BSA polymer. This fact was also observed before where a negative zeta potential was produced by a polymeric macromolecules indicating the presence of negative ionic groups in the backbone of the polymer.24 Therefore the negative zeta-potential before and after gamma-irradiation (Figures 4 and 5) indicates that the particle surface did not change significantly following gamma-irradiation. As a result, the particles would be dispersed in the same manner after gamma-irradiation. The shape, size and charge of the particle before and after gamma-irradiation indicate that the bovine serum albumin (BSA) polymer that made up the matrix of the particle was not destroyed. The BSA polymer, a heat sensitive material, has been shown to undergo denaturation under chemical and heat conditions.25 In this study, controlled gamma-irradiation of 25 kGy was sufficient to sterilize the particles while keeping the polymer intact.
In addition to the integrity of the polymer, it was also important to verify the integrity of the antisense because the functionality of the antisense as a drug depends on the antisense sequence to remain intact. To determine the stability of the antisense and the structural integrity, the antisense was evaluated by HPLC before and six months after applying gamma-irradiation. For the purpose of the HPLC analysis, the drug was extracted from the microspheres. Oligreen fluorescence assay was used for extraction. The HPLC profile of pure antisense showed a retention time at 14.5 min (Figure 6A). The peaks at retention times 2 min and 3 min are due to the solvent; these two peaks were observed when only the solvent was analyzed. The HPLC profile of antisense oligonucleotide extracted from the microspheres before the stability test and the gamma- irradiation showed the presence of similar peak at 14.5 min. A similar peak was observed at the same retention time of 14.5 min of antisense oligonucleotide which was extracted from the microsphere after the process of gamma-irradiation. Thus the presence of the peak at 14.5 min retention time, which is the characteristic peak of 18-mer NF-kB antisense in all three cases and also the lack of any other peak at other time points other than those contributed by solvent and blank microspheres indicated the absence of any detectable degradation of the antisense oligonucleotide in the samples (Figure 6B and 6C). The presence of the peak at retention time 14.5 min before and after gamma-irradiation clearly indicates that the antisense was not affected by the process of gamma-irradiation.
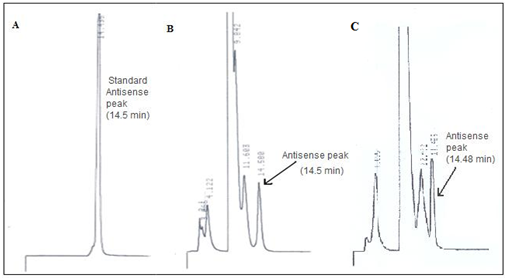
Figure 6 A. HPLC retention time peak of standard antisense oligonucleotide peak.
B. HPLC peak of antisense oligonucleotide extracted from the microsphere before applying gamma-irradiation.
C. HPLC peak of antisense oligonucleotide extracted from microsphere after applying gamma-irradiation.The antisense has the ability to block the cytokine activity. Therefore we demonstrated the TNF-α and IL-1B blocking activity acted by the antisense microsphere before and after gamma radiation. Equal concentration of antisense microsphere (200µg/mL) was used for biological activity comparison. The effect of microencapsulated antisense NF-kB on suppression of TNF-α and IL-1b depicted in Figure 7. TNF-α was suppressed 79 % by antisense microsphere before radiation, and 80% by the antisense microsphere group after gamma radiation. The solution group of antisense microsphere (604.4pg/mL) showed almost equal level of control group (670.4pg/mL) because the antisense in the solution is degraded without protection provided by polymeric microsphere. Without any protection the antisense has very short half-life and easily degraded in the system. On the other hand, in case of IL-1b the cytokine inhibition by antisense microsphere before and after gamma radiation was 58% and 60% respectively. In this case the solution group of antisense was almost equal to the control group for similar above mentioned reason. Apparently there were not much differences between the biological activity of gamma radiated antisense microsphere and non-radiated group to block cytokines.
Gamma-irradiation is the most commonly used sterilization process practiced in the pharmaceutical industry. Gamma-irradiation is required to sterilize micro particles for medical use. Although most commonly used and highly effective, gamma-irradiation may affect the drug and cause degradation. Antisense, a new class of drugs, may also be vulnerable to gamma-irradiation. The antisense is considered to be a next generation drug for its high specificity and effectiveness. The drug has very short half-life in the biological system and requires a protection from acidic and enzymatic degradation. Forming microspheres of the antisense is a very effective formulation for antisense, which requires to be sterilized by gamma-irradiation. The applying of gamma- irradiation may cause degradation of the polymer or the antisense drug itself in the particle. In this study, we have shown that an antisense drug encapsulated with albumin (BSA) polymer matrix is stable and biologically active following sterilization with gamma-irradiation.
None.
The author declares no conflict of interest.

©2016 Uddin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.