MOJ
eISSN: 2575-9094


Research Article Volume 2 Issue 4
1School of Life and Health Sciences, Aston University, England
2Department of Medicine, University of Tennessee Health Science Center, USA
3PNB Vesper Life Science PVT, Cochin, Kerala, India
Correspondence: Eric Lattmann, School of Life and Health Sciences, Aston University, England, Tel +44-(0)-121 204-3980
Received: June 28, 2018 | Published: July 6, 2018
Citation: Lattmann E, Russell ST, Singh M, et al. N–substituted 5–hydroxy–pyrrol–2–ones based cholecystokinin–2 antagonists as experimental anticancer agents for the treatment of lung cancer. MOJ Drug Des Develop Ther. 2018;2(4):179-188. DOI: 10.15406/mojddt.2018.02.00045
Background: Cholecystokinin and gastrin are endocrine growths factors for certain tumours and CCK1R and CCK2R receptors are ideal molecular targets for novel smart chemo‒therapeutics with a beneficial overall profile due to their anxiolytic and antidepressant properties. Lung cancers are fuelled by gastrin and therefore, selective gastrin (CCK2R) antagonists are ideal experimental drug candidates.
Objective: Synthesis and evaluation of novel CCK antagonists, most preferred CCK2 /gastrin selective for the treatment of lung cancers.
Methods: A fast and efficient synthesis of hydroxy‒pyrrolones in 2 steps from renewable biomass was performed. After initial radiolabelled receptor binding studies with hot CCK8, subsequent in vitro evaluation with isolated duodenum preparations confirmed CCK antagonism. Cell based studies using the MTT assay provided a candidate for in vivo xenograft models with nude mice. Rational drug design was supported by molecular modelling experiments.
Results: Potent and selective CCK antagonists were prepared as stable crystalline materials in high yields. Gastrin antagonists were in vitro active on isolated tissue preparations and inhibited breast, colon and lung cancer cell lines in vitro with IC50 to 45nM for the privileged hydroxyl‒pyrrolone lead structure in the MTT assay for human cancer cell lines. PNB‒101, a fluorinated 5‒hydroxy‒5‒aryl‒pyrrol‒2‒one, gave up to 80% inhibition of tumour growths by oral administration in athymic mice transplanted with the human lung cancer cell line H727.
Conclusion: PNB‒101 is a potential chemotherapeutic agent for CCK‒gastrin related cancers and entered preclinical development.
Keywords: cholecystokinin, pancreatic enzymes, proliferation, pancreatic cancer, anxiolytics, devazepide
CCK‒8 is the major circulating form of cholecystokinin.1 It is extensively found in the gastrointestinal tract and as a neuropeptide, it is widespread in the nervous system.2 Cholecystokinin, CCK, was originally identified to cause contractions of the gallbladder,3 later it was discovered as pancreozymin, triggering the release of pancreatic enzymes. In the end, after controversial studies, it was confirmed that cholecystokinin and pancreozymin are identical peptides.4 Cholecystokinin and its receptor has been extensively investigated as potential drug target.5 CCK‒agonists and CCK‒antagonists,6 have been clinically evaluated as therapeutics. In particular, CCK acted as growth factor in certain forms of cancer.7 Cholecystokinin caused proliferation in colon‒and pancreatic cancer cell lines and therefore, CCK‒antagonists were studied as growth factor inhibitors in these CCK sensitive cancers. According to the role of CCK as a neurotransmitter, CCK antagonists were investigated as anxiolytics,8 in the treatment of schizophrenia and satiety.9,10 Asperlicin was the first non‒peptidal CCK antagonist isolated from nature.11 The original lead structure was simplified by Merck and a fully synthetic antagonist Devazepide, was developed.12,13 This simplified antagonist was a potent and CCK1 selective cholecystokinin antagonist, containing a 1,4‒benzodiazepine template and an indole moiety. Proglumide was the first glutamic acid derivative, which was marketed as Milid for the treatment of ulcer. Lorglumide, was derived from proglumide, Figure 1 and is now a common experimental CCK1 receptor standard. The replacement of the indolyl amide group in devazepide by a urea linkage resulted in Merck’s CCK2 selective antagonist L‒365, 260. Further SAR optimization by Zeria from L‒365, 260 towards Z‒360 led to a CCK2–gastrin receptor antagonist with improved selectivity and bioavailability, which progressed into a phase 2 trial for pancreatic cancer.14‒18 The 1,4‒benzodiazepine structure was replaced by a non‒chiral pyrazol template in our early discovery programmes. A pyrazol, containing an indole carboxylic acid and a phenyl urea moiety, showed excellent anxiolytic and antidepressant properties in mice. Later, the 1,4‒benzodiazepine template was varied by a combinatorial solid phase synthesis and a simple n‒propyl derivative was found the best CCK antagonist. Key in our recent search, was the low molecular weight <350, an optimum logp value about 3 and a restricted polar surface area of less than 100A2, thus, providing us with molecules of a high membrane penetration. Aim of the drug discovery programme, initiated by PNB Vesper Life Sciences, was to systematically design from the 2(5H)‒furanone scaffold a hydroxyl‒pyrrolone scaffold with ligands for both CCK1 and CCK2 pathways. Initial results for CCK antagonists of the pyrrolone scaffold were communicated in the area of cancer therapeutics and inflammation. Here, a full biological evaluation of the PNB‒cancer molecules towards PNB‒101, a potent and selective fluorinated gastrin antagonist will be reported in detail with respect to the anti‒neoplastic properties of the molecule, in particular for lung cancer.19‒27
Synthesis
The chemicals were obtained from standard supplies as outlined in, NMR spectra were calibrated with TMS and the melting points were uncorrected.
Preparation of stage 1 intermediates
Synthesis of 3,4‒dichloro‒5‒phenyl‒5H‒furan‒2‒one, lactone A
Granulated aluminium chloride (20g, 0.15mol) was added slowly to a mixture of mucochloric acid (16.9g, 0.1mol) and arene (250ml). The reaction mixture was stirred overnight. It was then poured into a mixture of 100g ice and 32ml concentrated hydrochloric acid. The organic layer was separated and washed with 3x100ml water. The combined organic layers were dried over magnesium sulphate and the solvent was removed under vacuum. The oily residue was crystallized from n‒hexane.
Yield=70%; mp: 78‒79oC; MS (APCI(+)): (M+), 230/232 (M+1)m/z; 1HNMR (CDCl3) 250MHz: d= 7.22‒7.51 (m, 5H), 5.81 (s, 1H); 13CNMR (CDCl3):165.3, 152.2, 139.8, 130.5, 129.3, 128.5, 127.4, 127.2, 121.2, 83.5; IR (KBr‒disc) dmax: 3445, 3074, 3035, 2959, 2056, 1768, 1630, 1499, 1457 1294, 1224, 1028, 910, 772, 705cm‒1
3,4‒Dichloro‒5‒(4‒chloro‒phenyl)‒5H‒furan‒2‒one, lactone B
Yield=69% mp: 76‒78oC; MS (APCI(+)): 262/263/265 (M+)m/z; 1HNMR (CDCl3) 250MHz: d=7.48 (m, 2H), 7.35(m, 2H), 5.91(s, 1H); 13CNMR (CDCl3) 165.3, 152.0, 136.6, 130.1, 129.6, 128.7, 121.3, 82.9; IR (KBr‒disc) dmax: 3451, 3075, 2952, 2051, 1769, 1636, 1497, 1419, 1289, 1231, 1027, 927, 826, 748, 720cm‒1
3,4‒Dichloro‒5‒(4‒fluoro‒phenyl)‒5H‒furan‒2‒one, Lactone C
Yield=79% mp: 74‒76oC; MS (APCI(+)): 212/213/214 (M+1), 1HNMR (CDCl3) 250MHz: d=7.46(m, 2H), 7.34 (m, 2H), 5.90(s, 1H); 13CNMR (CDCl3) 165.0, 151.9, 136.4, 130.1, 129.6, 128.7, 121.3, 82.7; IR (KBr‒disc) dmax: 3450, 3075, 2952, 2049, 1768, 1636, 1497, 1419, 1289, 1027, 927, 826, 749, 719cm‒1.
Preparation of stage 2 products: pyrrolone synthesis
The general method was previously reported and the spectroscopic data are reported for a full SAR analysis and optimisation with respect to antineoplastic properties.
4‒Chloro‒5‒hydroxy‒1‒methyl‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 1
Yield=75%; mp: 146‒148oC; MS (APCI(+)): 193/195(M+1), 224/226(M+) m/z; 1HNMR (DMSO‒d6)) 250 MHz: 7.29‒7.48 (m, 5H), 6.49(s, 1H), 2.08 (s, 3H) 13CNMR (CDCl3) 168.1, 156.4, 134.1, 129.4, 128.9, 126.2, 121.3, 92.6, 24.5ppm. IR (KBr‒disc): 3224, 3110, 2952, 2820, 2617, 2375, 2339, 1975, 1697, 1605, 1453, 1438, 1258, 1207, 1065, 992, 856, 764, 704cm‒1.
4‒Chloro‒5‒(4‒chloro‒phenyl)‒5‒hydroxy‒1‒methyl‒1,5‒dihydro‒pyrrol‒2‒one 2
Yield=66%; mp: 179‒181oC; MS (APCI(+)): 227/229/231 (M+1), 258/260/262(M+) m/z; 1HNMR (CDCl3) 250MHz: 7.31‒7.42 (m, 4H), 6.06 (s, 1H), 4.56‒4.71 (bs, 1H), 2.60 (s, 3H) 13CNMR (CDCl3) 167.8, 156.0, 135.5, 132.8, 129.1, 127.8, 121.6, 92.2, 24.4ppm. IR (KBr‒disc) 3429, 3102, 2970, 2932, 2857, 1677, 1611, 1494, 1475, 1431, 1202, 1151, 1091, 988, 928, 811, 692cm‒1.
4‒Chloro‒5‒hydroxy‒1‒isopropyl‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 3
Yield=79%; mp: 163‒165oC; MS (APCI(+)): 193/195(M+1), 252/254(M+) m/z; 1HNMR (CDCl3) 250MHz: 7.40‒7.51 (m, 5H), 6.14 (s, 1H), 3.81 (bs, 1H), 3.42 (m, 1H), 1.33& 1.21 (m, 6H) 13C NMR (CDCl3) 167.5, 155.0, 135.0, 129.1, 128.5, 126.4, 122.4, 93.4, 45.6, 21.1, 20.0ppm. IR (KBr‒disc) 3227, 2990, 2940, 2365, 2350, 1956, 1693, 1615, 1456, 1428, 1247, 1131, 1072, 1009, 934, 847, 747, 697cm‒1.
4‒Chloro‒5‒(4‒chloro‒phenyl)‒5‒hydroxy‒1‒isopropyl‒1,5‒dihydro‒pyrrol‒2‒one 4
Yield =69%; mp: 127‒130oC; MS (APCI(+)): 286/288/290 (M+) m/z; 1HNMR (CDCl3) 250MHz: 7.31(m, 4H), 6.06 (s, 1H), 3.33 (m, 1H), 1.25&1.10 (m, 6H). 13CNMR (CDCl3) 167.1, 154.0, 136.7, 133.4, 128.9, 128.0, 123.2, 92.9, 45.6, 20.1, 21.3ppm. IR (KBr‒disc) 3272, 2978, 2927, 1691, 1614, 1496, 1429, 1384, 1352, 1249, 1096, 1012, 936, 846, 801, 683cm‒1.
4‒Chloro‒1‒cyclopropyl‒5‒hydroxy‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 5
Yield=83%; mp: 177‒179oC; MS (APCI(+)): 193/195 (M+1), 250/252 (M+) m/z; 1HNMR (CDCl3) 250MHz: d= 7.41 (m, 5H), 6.09 (s, 1H), 3.50 (m, 1H), 2.18 (m, 1H), 0.95& 0.38 (m, 4H); 13CNMR (CDCl3) 167.4, 154.8, 135.2, 129.2, 128.8, 126.1, 122.2, 93.5, 22.6, 3.8, 5.1ppm. IR (KBr‒disc) 3416, 3260, 3105, 3011, 2363, 2338, 1671, 1602, 1490, 1450, 1409, 1369, 1256, 1144, 1032, 939, 833, 752, 702cm‒1.
4‒Chloro‒5‒(4‒chloro‒phenyl)‒1‒cyclopropyl‒5‒hydroxy‒1,5‒dihydro‒pyrrol ‒2‒one 6
Yield=72%; mp: 169‒171oC; MS (APCI(+)): 284/286/288 (M+) m/z; 1HNMR (CDCl3) 250MHz: 7.22 (m, 4H), 5.97 (s, 1H), 3.98 (bs, 1H), 1.76 (m, 1H), 0.24‒0.99 (m, 4H); 13CNMR (CDCl3) 165.8, 155.4, 144.2, 133.7, 129.0, 127.7, 122.2, 91.7, 22.6 , 3.7, 5.2ppm. IR (KBr‒disc) 3433, 3220, 3019, 2935, 2858, 1700, 1675, 1497, 1412, 1251, 1209, 1144, 1089, 1015, 940, 844, 802, 679cm‒1.
4‒Chloro‒5‒hydroxy‒1‒isobutyl‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 7
Yield=85%; mp: 167‒169oC; MS (APCI(+)): 266/268 (M+) m/z; 1HNMR (CDCl3) 250MHz: 7.38‒7.51 (m, 5H), 6.24 (s, 1H), 4.79 (bs, 1H), 3.23&2.18 (m, 2H), 1.71 (m, 1H), 0.76 (m, 6H) 13CNMR (CDCl3) 168.5, 155.7, 137.1, 129.2, 128.7, 126.2, 121.7, 93.1, 47.6, 27.5, 20.4ppm. IR (KBr‒disc) 3237, 3114, 2965, 2926, 2881, 2374, 2343, 1675, 1614, 1460, 1416, 1299, 1251, 1202, 1150, 1072, 1027, 878, 758, 696cm‒1.
4‒Chloro‒5‒(4‒chloro‒phenyl)‒5‒hydroxy‒1‒isobutyl‒1,5‒dihydro‒pyrrol‒2‒one 8
Yield = 66%; mp: 155‒158oC; MS (APCI(+)): 300/302/304 (M+) m/z; 1HNMR (CDCl3) 250MHz: 7.30 (m, 4H), 6.19 (s, 1H), 3.13 (m, 1H), 2.49 (m, 1H), 1.69 (m, 1H), 0.69 (t, J = 4.5 Hz, 6H) 13CNMR (CDCl3) 163.3, 156.3, 139.4, 134.8, 129.1, 127.7, 122.3, 95.0, 47.6, 27.6, 20.4ppm. IR (KBr‒disc) 3426, 3252, 2964, 2850, 1684, 1406, 1209, 1095, 817, 743, 703cm‒1.
4‒Chloro‒5‒(4‒fluoro‒phenyl)‒5‒hydroxy‒1‒isobutyl‒1,5‒dihydro‒pyrrol‒2‒one 9Yield=76%; mp: 158‒159oC; MS (APCI(+)): 300/302/304 (M+) m/z; 1HNMR (CDCl3) 250MHz: 7.30 (m, 4H), 6.19 (s, 1H), 3.13 (m, 1H), 2.49 (m, 1H), 1.69 (m, 1H), 0.69 (t, J = 4.5 Hz, 6H) 13CNMR (CDCl3) 163.3, 156.3, 139.4, 134.8, 129.1, 127.7, 122.3, 95.0, 47.6, 27.6, 20.4ppm. IR (KBr‒disc) 3426, 3252, 2964, 2850, 1684, 1406, 1209, 1095, 817, 743, 703cm‒1.
4‒Chloro‒1‒cyclopentyl‒5‒hydroxy‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 11
Yield=81%; mp: 180‒182oC; MS (APCI(+)): 278/280 (M+) m/z; 1HNMR (CDCl3) 250MHz: d= 7.51 (m, 5H), 6.08 (s, 1H), 4.87 (bs, 1H), 3.59 (m, 1H), 1.99 (m, 2H), 1.81 (m, 4H), 1.46 (m, 4H); 13C NMR (CDCl3) 167.2, 155.0, 135.2, 129.1, 128.6, 126.5, 122.2, 93.3, 54.3, 30.0, 28.8, 24.5, 24.4ppm. IR (KBr‒disc) 3220, 2961, 2877, 2373, 2341, 1684, 1613, 1448, 1426, 1248, 1199, 1141, 1070, 934, 850, 750, 701cm‒1.
4‒Chloro‒5‒(4‒chloro‒phenyl)‒1‒cyclopentyl‒5‒hydroxy‒1,5‒dihydro‒pyrrol‒2‒one 12
Yield=73%; mp: 157‒159oC; MS (APCI(+)): 312/314/316 (M+) m/z; 1HNMR (CDCl3) 250MHz: d= 7.42 (m, 4H), 6.03 (s, 1H), 4.99 (bs, 1H), 3.51‒3.62 (m, 1H), 1.97‒2.19 (m, 2H), 1.68‒1.93 (m, 8H); 13C NMR (CDCl3) 167.1, 154.8, 135.2, 133.9, 128.9, 128.0, 122.3, 93.0, 54.3, 30.0, 28.9, 24.5ppm. IR (KBr‒disc) 3407, 3276, 2968, 2922, 2883, 2379, 2339, 1691, 1491, 1429, 1367, 1249, 1203, 1092, 1013, 932, 843, 787, 709cm‒1.
4‒Chloro‒1‒hexyl‒5‒hydroxy‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 13
Yield=51%; mp: 173‒175oC; MS (APCI(+)):294/296 (M+) m/z; 1H NMR (CDCl3) 250MHz: 7.40 (m, 5H), 6.15 (s, 1H), 4.76 (bs, 1H), 3.28 (m, 1H), 2.91 (m, 1H), 1.09‒1.59 (m, 8H), 0.78‒0.92 (t, J = 7.1 Hz, 3H) 13C NMR (CDCl3) 168.0, 155.6, 134.9, 129.2, 128.7, 126.2, 121.8, 93.0, 40.2, 31.3, 28.7, 26.8, 22.5, 14.0ppm. IR (KBr‒disc) 3245, 2930, 2865, 1689, 1658, 1494, 1453, 1412, 1365, 1321, 1150, 1069, 927, 753, 696 cm‒1.
4‒Chloro‒5‒(4‒chloro‒phenyl)‒1‒hexyl‒5‒hydroxy‒1,5‒dihydro‒pyrrol‒2‒one 14
Yield=49%; mp: 169‒172oC; MS (APCI(+)): 328/330/332 (M+) m/z; 1H NMR (CDCl3) 250MHz: 7.31(m, 4H), 6.15 (s, 1H), 3.24 (m, 1H), 2.67 (m, 1H), 1.04‒1.69 (m, 8H), 0.74 (t, J = 6.3 Hz, 3H) 13C NMR (CDCl3) 165.8, 155.7, 140.8, 136.9, 129.1, 127.8, 91.6, 40.3, 30.8, 29.1, 26.8, 22.6, 15.2ppm. IR (KBr‒disc) 3446, 2935, 2863, 1698, 1413, 1252, 1200, 1138, 1092, 1013, 938, 846, 814, 702cm‒1.
4‒Chloro‒1‒cyclohexyl‒5‒hydroxy‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 15
Yield=57%; mp: 170‒172oC; MS (APCI(+)): 292/294 (M+) m/z; 1HNMR (CDCl3) 250MHz: 7.26‒7.61 (m, 5H), 6.08 (s, 1H), 3.77 (bs, 1H), 2.88 (m, 1H), 1.21‒2.07 (m, 10H); 13CNMR (CDCl3) 163.9, 153.9, 135.0, 129.25, 128.9, 126.4, 122.9 , 96.0, 53.6, 32.8, 31.1, 29.8, 26.2 , 24.2ppm. IR (KBr‒disc) 3440, 2924, 2858, 2355, 2344, 1641, 1449, 1367, 1250, 1138, 1016, 996, 742, 695cm‒1.
4‒Chloro‒1‒(phenyl)‒5‒hydroxy‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 16
Yield=48%; mp: 168‒171oC; MS (APCI(+)): 314/316 (M+) m/z; 1HNMR (CDCl3) 250MHz: d=7.46 (m, 5H), 7.34 (m, 5H), 6.38 (s, 1H), 3.68 (bs, 1H); 13CNMR (CDCl3) 168.9, 159.7, 136.9, 135.1, 132.4, 129.9, 129.0, 126.9, 123.0, 122.3, 122.2, 93.5; IR (KBr‒disc) 3517, 3357, 3114, 2840, 2674, 2361, 2342, 1678, 1607, 1464, 1412, 1361, 1208, 1138, 1071, 988, 755, 700cm‒1.
1‒Benzyl‒4‒chloro‒5‒hydroxy‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 17
Yield=71%; mp: 165‒167oC; MS (APCI(+)): 300/302 (M+) m/z; 1HNMR (CDCl3) 250MHz: 7.36 (m, 5H), 7.24 (m, 5H), 6.08 (s, 1H), 4.69 (m, 2H), 3.62 (bs, 1H); 13CNMR (CDCl3) 167.9, 155.9, 137.6, 134.4, 129.3, 128.7, 128.4, 128.4, 127.3, 127.1, 126.4, 93.2, 43.4; IR (KBr‒disc) 3446, 3279, 3098, 2931, 2850, 2374, 2334, 1684, 1611, 1456, 1413, 1349, 1276, 1205, 1128, 1051, 696cm‒1.
1‒Benzyl‒4‒chloro‒5‒(4‒chloro‒phenyl)‒5‒hydroxy‒1,5‒dihydro‒pyrrol‒2‒one 18
Yield=59%; mp: 149‒152oC; MS (APCI(+)): 334/336/338 (M+) m/z; 1H NMR (CDCl3) 250MHz: d= 7.33 (m, 4H), 7.16 (m, 5H), 6.09 (s, 1H), 4.60 (m, 2H), 13CNMR (CDCl3) 167.6, 155.4, 137.5, 135.3, 133.2, 129.1, 129.0, 128.9, 128.6, 128.4, 127.9, 127.4, 121.9, 92.6, 43.2; IR (KBr‒disc) 3442, 2931, 2849, 2365, 2339, 1674, 1616, 1492, 1406, 1349 1272, 1199, 1094, 1018, 817, 699cm‒1.
1‒Benzyl‒4‒chloro‒5‒(4‒fluoro‒phenyl)‒5‒hydroxy‒1,5‒dihydro‒pyrrol‒2‒one 19
Yield = 59%; mp: 149‒152oC; MS (APCI(+)): 318/319/320 (M+) m/z; 1H NMR (CDCl3) 250MHz: d= 7.32 (m, 4H), 7.14 (m, 5H), 6.10 (s, 1H), 4.60 (m, 2H), 13CNMR (CDCl3) 167.5, 155.2, 137.2, 135.3, 133.2, 129.0, 129.0, 128.6, 127.6, 128.4, 127.9, 127.4, 121.9, 92.6, 43.2; IR (KBr‒disc) 3442, 2931, 2849, 2365, 2339, 1674, 1616, 1492, 1406, 1349 1272, 1199, 1094, 1018, 817, 699cm‒1.
4‒Chloro‒5‒hydroxy‒5‒phenyl‒1‒((S)‒(‒)‒1‒phenyl‒ethyl)‒1,5‒dihydro‒pyrrol‒2‒one 20
Yield=66%; mp: 162‒164oC; MS (APCI(+)): 314/316 (M+)m/z; 1HNMR (CDCl3) 250MHz: d= 7.44 (m, 7H), 7.08‒7.25 (m, 3H), 5.96 (s, 1H), 4.16 (m, 1H), 3.37 (bs, 1H), 1.49 (m, 3H); 13CNMR (CDCl3) 167.3, 154.3, 142.5, 134.7, 129.4, 128.7, 128.4, 127.7, 127.3, 126.4, 123.0, 93.8, 53.5, 18.8ppm. IR (KBr‒disc) 3241, 2983, 2932, 2863, 2366, 2347, 1686, 1661, 1614, 1494, 1456, 1425, 1356, 1258, 1202, 1025, 931, 855, 755, 692cm‒1.
4‒Chloro‒5‒hydroxy‒5‒phenyl‒1‒((S)‒(‒)‒1‒phenyl‒ethyl)‒1,5‒dihydro‒pyrrol‒2‒one 21
Yield=4%; MS (APCI(+)): 314/316 (M+) m/z; 1HNMR (CDCl3) 250MHz: d= 7.29‒7.53 (m, 7H), 6.95 (m, 3H), 6.08 (s, 1H), 4.78 (m, 1H), 2.71 (bs, 1H), 1.63 (m, 3H).
4‒Chloro‒5‒hydroxy‒1‒phenethyl‒5‒phenyl‒1,5‒dihydro‒pyrrol‒2‒one 22
Yield=89%; mp: 155‒158oC; MS (APCI(+)): 314/316 (M+) m/z; 1HNMR (CDCl3) 250MHz: d= 7.09‒7.53 (m, 10H), 6.20 (s, 1H), 3.74 (m, 1H), 2.88‒3.29 (m, 3H), 2.65 (m, 1H); 13CNMR (CDCl3) 168.0, 155.7, 139.0, 134.6, 129.4, 128.85, 128.84, 128.6, 126.6, 126.2 , 121.8, 92.7, 41.9 , 34.6ppm. IR (KBr‒disc) 3433, 3246, 2929, 2366, 2334, 1681, 1658, 1607, 1455, 1406, 1251, 1151, 1128, 1066, 931, 753, 699cm‒1.
4‒Chloro‒5‒(4‒chloro‒phenyl)‒5‒hydroxy‒1‒phenethyl‒1,5‒dihydro‒pyrrol‒2‒one 23
Yield=45%, mp: 145‒148oC; MS (APCI(+)): 348/350/352 (M+)m/z; 1H NMR (CDCl3) 250MHz: d= 7.22‒7.49 (m, 7H), 7.12‒7.18 (m, 2H), 6.13 (s, 1H), 3.68 & 2.64 (m, 2H), 2.88 (m, 2H); 13C NMR (CDCl3) 250MHz: 167.7, 155.5, 138.8, 135.5, 133.3, 129.1, 128.8, 128,7, 127.7, 126.7, 121.9, 92.3, 42.0, 34.5; IR (KBr‒disc) 3421, 3228, 2925, 2848, 2370, 2338, 1684, 1658, 1606, 1461, 1406, 1248, 1190, 1097, 935, 806, 697cm‒1.
4‒Chloro‒5‒(4‒fluoro‒phenyl)‒5‒hydroxy‒1‒phenethyl‒1,5‒dihydro‒pyrrol‒2‒one 24
Yield = 55%, mp: 147‒151oC; MS (APCI(+)): 332/334 (M+) m/z; 1H NMR (CDCl3) 250MHz: d= 7.22‒7.49 (m, 7H), 7.12‒7.18 (m, 2H), 6.13 (s, 1H), 3.68 & 2.64 (m, 2H), 2.88 (m, 2H); 13CNMR (CDCl3) 250MHz: 167.7, 155.5, 138.8, 135.5, 133.3, 129.1, 128.8, 128,7, 127.7, 126.7, 121.9, 92.3, 42.0, 34.5; IR (KBr‒disc) 3421, 3228, 2925, 2848, 2370, 2338, 1684, 1658, 1606, 1461, 1406, 1248, 1190, 1097, 935, 806, 697cm‒1.
I‒CCK‒8 Radioligand cholecystokinin binding assay
CCK2 and CCK1 receptor binding assays were performed using standard receptor binding assays.24,25 Male guinea pig brain tissues were prepared according to the modified method described.28 Pancreatic membranes were prepared as described and binding assays were carried out with L‒363, 260 as standard.29
Isolated tissue preparations
Male Sprague Dawley rats, weighing 200‒250g were used and all animal care and experimental protocols adhered to the relevant laws and guidelines of the institution. The animals were housed under standard conditions of temperature (25°C) with unrestricted access to food and water. Experimental details are reported in using Tyrode solution and CCK‒5, as agonist at increasing concentrations in Tyrode solution Figure 2. Test molecules and lorglumide as standard, were added to the organ bath with a 10minute incubation period prior to the addition of CCK.
Molecular modeling
Protein structures such as 1HZN for the cholecystokinin receptor and 1L4T for the gastrin receptor were downloaded from the protein data bank in pdb format. Guest‒host‒interactions were analysed with Autodock Vina and after convergence was achieved, the docking results were visualized with Designer studio 4.5.
In vivo study
Xenograft study in NSG mice
Human cancer cells were grown in DMEM supplemented with 10% FBS at 37oC in an incubator with 5%CO2. For xenograft implantation the relevant cells were harvested and viable cells were determined by trypan blue exclusion. A cell suspension in growth medium was prepared and this cell suspension in growth medium was implanted subcutaneously in NSG mice Figure 3. 1million cells were transplanted subcutaneously per mouse. Once tumours reached 100mm3, the animals were randomized within the respective cell line and treated orally. Animals were sacrificed at the end of the experiment or when the tumours reached over 1500mm3 or when the animals lost over 20% body weight. All experiments were performed in compliance with the relevant laws and institutional guidelines and the institutional bioethics committee has approved the experiments.
Statistical methods
ANOVA was the selected one‒way analysis of variance and a difference was indicated as significant when the difference was found p<0.05.
Chemistry
5‒arylated dichloro‒2(5H)‒furanones A‒C were synthesised from mucochloric acid (Scheme 1). Mucochloric acid is commercially available under oxidising conditions with hydrochloric acid from furfural. Furfural is available from biomass by hydrolysis with strong acids. Mucochloric acid was reacted into the stage 1 intermediates with benzene, chlorobenzene and fluorobenzene Figure 4. The arene or haloarene acted as reagent and solvent at RT under the development of hydrogen chloride gas. Depending on the scale of the reaction the exothermic properties of the electrophilic substitution reaction required cooling with ice. For the Friedel‒ Crafts‒Acylation the best catalyst and Lewis acid is granulated aluminium chloride on a small scale. However, on a larger scale aluminium chloride was replaced by trifluoroborane in THF to closely control the exothermic nature of the reaction Figure 5A & 5B. The 5‒arylated 3,4‒dichloro‒2 (5H)‒furanones A, B & C (Stage 1 intermediates) reacted in ether with alkyl‒ and aryl alkyl amines into N‒alkylated hydroxyl‒pyrrolones 1‒24 ( Stage 2 products ). Diethylether or MTB ether is essential to furnish the stage 2 products in high yields under mild conditions and 2 step synthesis is outlined in Scheme 1. All pyrrolones 1‒24 are present in the 5‒membered ring form, as a hydroxyl‒pyrrolone and not in the ring opened keto form. The 5‒arylated 2(5H)‒furanones reacted selectively in the ester position ‒ a lactone was converted into a lactam‒and no attack in the 4‒position, Michael position, was observed. Previously, using a polar solvent, such as dimethylformamide, DMF, the IPSO substitution in the 4‒position was described for pseudo‒ esters.30 In terms of reaction mechanism a ring‒opening ring‒closure mechanism is proposed here for the formation of the hydroxy‒pyrrolones. It is supposed, that the first step in the reaction sequence, is a ring opening of the stage 1 intermediate with amide formation. Subsequently, the keto form of the acyclic amide was converted in situ into a lactam under the elimination of hydrogen chloride. During this process, involving an achiral arylated ketoform, the stereo chemical information is lost and experimentally a racemic mixture is found of the R‒and S‒enantiomeric forms. A 50:50 ratio of both enantiomers was found by chiral HPLC in solution in methanol for lactam 7 and 22 Figure 6. The phenylfuranones were evaluated previously as anticancer agents based on their alkylating properties and not as a targeted chemotherapeutic agent. Overall the desired N‒alkylated 5‒phenyl pyrrolones 1‒24 were obtained in only a 2 stage process as mostly white and crystalline materials.31
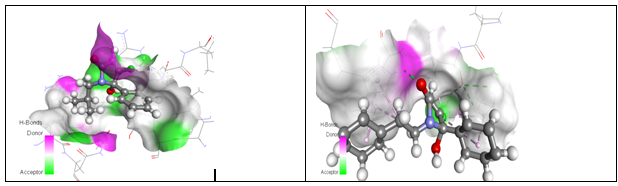
Figure 5 Figure 5(A) Drug receptor interactions of isobutyl lactam 7 and the CCK1 receptor. Figure 5(B) Docking of CCK antagonist lactam 22 into the CCK2 receptor.
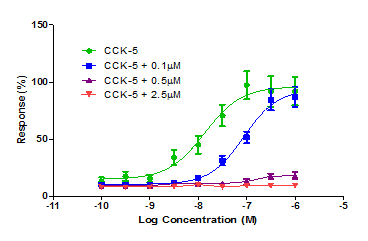
Figure 6 Log concentration‒ response curves obtained in the presence of CCK‒8S alone and CCK‒8S in the presence of lactame 24 (PNB‒101).
SAR optimisation
The first step was to screen for potent binding affinity and to identify a CCK1 or CCK2‒selective ligand for subsequent m‒1and in vivo evaluation. Using radiolabelled iodinated cholecystokinin, inhibition of binding was determined for all test molecules and the IC50 are outlined in Table 1. Lorglumide served as CCK1 standard and L‒365,260 was used as CCK2 standard. Hydroxy‒pyrrolones containing an N‒methyl group, lactame 1‒2, showed no binding affinity and the formation of homologues resulted in micromolar binding affinity for the iso‒propyl group, as seen in lactame 3 and 4. Ring closure on the N‒substituent, from isopropyl to cyclo‒propyl resulted in a loss of activity (lactame 5, 6) and the ring enlargement from a 3‒membered to a 5‒membered ring system (entry 11‒12) resulted in a similar binding affinity. Changing N‒propyl into an N‒butyl resulted in a major increase of binding activity and the overall best substituent was found iso‒butyl on the central nitrogen atom, derivative 7. The introduction of a halogen atom into the para‒position of the phenyl group resulted in an increase of binding affinity as observed for lactam 8 and lactam 9. The iso‒butyl group on the N‒atom produced the best overall ligand, with CCK1 selectivity and the IC50 was reduced significantly for t‒butyl derivative 10. The n‒pentyl analogue was formed in very low yields and the n‒hexyl lactams 13, 14 clearly lost binding affinity and the same was observed for the cyclohexyl derivative 15. A phenyl substituent on the N in pyrrolone 16, resulted in a loss of activity (>10mM). The N‒benzylated derivative pyrrolone 17, resulted in non‒selective CCK‒ligand. A chlorine atom, changed the binding affinity marginally for the lactam 18 and the introduction of fluorine for lactam 19, resulted in a significantly improved binding affinity, thus providing us with a potent mixed CCK antagonist. The introduction of a chiral amine, such as methyl benzyl amine provided diastereoisomers (Entry 20, 21), which were separated by column chromatography, and both stereoisomers occurred a lower affinity than the parent benzyl derivative 17. The introduction of a single spacer, such as a methylene group, resulted a highly selective CCK2 ligand 22, which is 450times selective for the CCK2/gastrin receptor. Chlorination in the p–position, lactam 23, did not enhance binding affinity any further, but a fluorine atom enhanced binding affinity towards for the gastrin receptor to an IC50 as low as 13nM. Thus, lactam 24, was identified a SAR optimised molecule, possibly as a result of an additional hydrogen bond of the fluorine atom with the gastrin receptor Table 1.
Overall, the introduction of alkyl groups, most preferred an isobutyl‒group, provided a CCK1 selective antagonist 9, which was the selected development candidate PNB‒028 for colon and pancreatic cancer. The N‒benzylated pyrrolone 17 displayed a non‒selective receptor binding profile and the modification of the spacer between the N‒atom of the central pyrrolone template (Lactam 22 and 24) from benzyl to phenyl‒ethyl provided us with potent and selective CCK2 receptor antagonists Figure 2. These selected molecules were evaluated in vitro using tissue based assays. Molecular modelling was performed to rationalise the drug receptor interactions for the parent molecules. Molecular modelling studies were performed for isobutyl derivative with the CCK1 receptor Figure 3. The isobutyl group of the ligand 7 interacted with a hydrophobic cave of the receptor, centred at Ala‒14. The carbonyl group in the 2‒position bond via hydrogen binding towards the CCK receptor with Arg‒9 and the N‒atom of the lactam interacted with Glu‒17. The 5‒hydroxy‒group of the ligand displayed interactions with of Asn‒6, while the phenyl group has no interaction with tryptophan or phenylalanine. Pi‒alkyl interactions only may explain the small increase in binding affinity of the chlorinated analogue compared with PNB‒081, based on interaction with Leu‒29 and Ile‒28. If the para phenyl position is not blocked by the chorine atom, most interestingly, the proposed metabolite can interact with the CCK2 receptor. It is supposed that lactam 7, is hydroxylated in the para phenyl position by P450 and this metabolite may interact with the CCK2 receptor via His 122 interaction. Most interestingly hydroxylation may also enhance CCK1 affinity due to interactions with Arg 9. The docking of lactam 22 into the CCK2 receptor is outlined in and some key interactions are highlighted for one final pose of minimal energy Figure 7A & 7B. The 5‒hydroxy group of the central pyrrolone template interacted via hydrogen binding with the N group of Trp114. The phenyl group of the N‒phenylethyl‒ side chain bound to the aromatic indole system of Trp114 and electron withdrawing groups may enhance these aromatic interactions. The lipophilic pocket allowed principally a wide range of substituents, but only phenyl and not cyclo‒hexyl could be realised synthetically. The 5‒phenyl group of the pyrrolone template bound via Ile 184 and Leu 133, based on van der Waals interactions and not aromatic interactions. The introduction of electron withdrawing groups, such as halogen atoms, is therefore only marginally enhancing binding affinity. The Fluorinated derivative 24 may feature hydrogen binding with His144 and this extra interaction may explain the improved affinity of lactam 24 over 23. Overall, the observed binding affinity and the predicted interaction based on molecular modelling interaction correlated well for the cholecystokinin and the gastrin receptor.
Isolated tissue preparations
A clear SAR was obtained for the entire series of molecules, outlined in Table 1, with respect to gastrin selectivity. The fluorinated lactame 24 was selected for in vitro evaluation using isolated tissue preparations. Using the isolated rat duodenum preparation, stable amplitude was generated for pentagastrin and a reduction of this amplitude was observed dose dependently for lactam 24, which is outlined in Figure 4. This assay represented a fast and efficient way to screen for CCK antagonists using classical isolated tissue preparations. The selected lactam acted as much lower concentrations than L‒365, 260, the CCK2 standard. It appeared Figure 4 that the effect of the antagonist 24 in the rat duodenum assay is insurmountable and an irreversible inhibitor is ideal for the development of antineoplastic agents. The function of the fluorine atom in lactam 24 was found dual to firstly enhance binding affinity and secondly to block metabolism of the molecules in the para–phenyl position, thus enhancing oral bioavailability. From 24 molecules in Table 1 the phenyl‒ethyl substituent was identified a privileged structure. Together with the isobutyl and benzyl derivatives a series of close analogues were screened in vitro using cell based assays.
Lactam |
X= |
R= |
CCK1[mM] |
CCK2[mM] |
1 |
H |
Methyl‒ |
2.5±0.2 |
>10 |
2 |
Cl |
Methyl‒ |
2.0±0.2 |
>10 |
3 |
H |
Isopropyl‒ |
0.2±0.02 |
0.9±0.03 |
4 |
Cl |
Isopropyl‒ |
0.3±0.04 |
3.7±0.4 |
5 |
H |
Cyclopropyl‒ |
7.5±0.4 |
>10 |
6 |
Cl |
Cyclopropyl‒ |
4.0±0.2 |
>10 |
7 |
H |
Isobutyl‒ |
0.020±0.01 |
1.2±0.3 |
8 |
Cl |
Isobutyl‒ |
0.008±0.01 |
0.4±0.2 |
9 |
F |
Isobutyl‒ |
0.012±0.01 |
0.75±0.2 |
10 |
H |
t‒butyl‒ |
0.12±0.22 |
0.9±0.03 |
11 |
H |
Cyclopentyl‒ |
0.36±0.03 |
0.84±0.2 |
12 |
Cl |
Cyclopentyl‒ |
2.5±0.03 |
>10 |
13 |
H |
Hexyl‒ |
4.5±0.3 |
>10 |
14 |
Cl |
Hexyl‒ |
3.6±0.3 |
>10 |
15 |
H |
Cyclohexyl‒ |
2.5±0.3 |
>10 |
16 |
H |
Phenyl‒ |
>10 |
>10 |
17 |
H |
Benzyl‒ |
0.85±0.03 |
0.020±0.005 |
18 |
Cl |
Benzyl‒ |
0.51±0.004 |
0.022±0.004 |
19 |
F |
Benzyl‒ |
0.11±0.004 |
0.012±0.004 |
20 |
H |
Methylbenzyl‒ |
0.6±0.04 |
0.021±0.01 |
21 |
H |
Methylbenzyl‒ |
0.42±0.03 |
0.21±0.05 |
22 |
H |
Phenylethyl‒ |
>10 |
0.022±0.002 |
23 |
Cl |
Phenylethyl‒ |
>10 |
0.030±0.001 |
24 |
F |
Phenylethyl‒ |
>10 |
0.013±0.001 |
Lorglumide |
‒ |
‒ |
0.17±0.01 |
>10 |
L‒356,260 |
‒ |
‒ |
0.25±0.01 |
0.003±0.001 |
Table 1 CCK binding affinity expressed in IC50 in micromolar using iodinated hot CCK8 as radioligands with cortex and pancreatic membranes; N=3
Cell based in vitro assay
CCK antagonists are associated with an array of therapeutic applications, but the focus of our research programme was to provide a non‒toxic, orally available CCK antagonist for the treatment of cancers. The antineoplastic properties of selected NCE and standard CCK antagonists, such as L‒365,260 (CC1H) and lorglumide (CCK1) were subsequently investigated, using a range of CCK associated cancer cell lines. It was screened for an inhibitor of viability in certain CCK related cancer cell lines using the MTT assay. MCF‒7 and MB‒231 are humane breast cancer cell lines and MCF‒7 did not express CCK receptors. For MCF‒7 no activity was found in vitro for the test molecules and standards. The triple resistant cell line MDA‒MB‒231 showed inhibition in vitro in our experiments and in xenografts in nude mice, antineoplastic activity was associated with the gastrin releasing peptide receptor independent from oestrogen. MAC 13 and MAC16 cell lines are murine colon cancer cell lines. MAC16 is resistant to alkylating agents and has a high expression of the CCK receptor In line with the receptor expression, activity was found for lactame 7 and 9, but the IC50 for benzylated lactame.32,33 19 was 4 times higher. Previously we reported activity for lactame 9 on pancreatic cancer cell lines. Most interestingly, no other CCK antagonist, such as L‒365, 260 or lorglumide was found active for this cell line, which is, when transplanted into mice lethal within 7 days. Based on this key difference between the known agents and our inhibitors, it may be concluded, that the unsurmountable irreversible properties of the antagonists are blocking only the proliferation of cell growth. The anticancer activity of the agents is not limited to GI cancers. The cytotoxicity of 2 selected human lung cancer cell lines was shown initially for lactam 19. The mixed CCK antagonist 19 showed a low nanomolar activity in cell based assays. Lung cancer is associated with overexpression of CCK2 receptors.34 NCI‒H322 was derived in 1981 from a primary bronchioalveolar carcinoma of the lung from a 52 year old male. Cells of this non‒small cell carcinoma cell line produce tumours in athymic mice. H‒727, the second human lung cancer cell line in our programme, was studied earlier for the gastrin CCK2 antagonist CI‒988 and proliferation was promoted by CCK8S and gastrin. Based on binding affinity, results from isolated tissue preparations and the experience of fluorinated molecules having favourable bioavailability, lactam 24 was submitted to xenograft analysis using the humane H‒727 cell line (Table 2).
Breast cancer |
Colon cancer |
Lung cancer |
||||
IC50 [nM] |
MCF‒7 |
MDA‒MB‒231 |
MAC13 |
MAC16 |
NCI‒H322 |
H‒727 |
L‒365,260 |
>5000 |
941±88 |
>5000 |
>5000 |
441±54 |
841±88 |
Lorglumide |
>5000 |
>5000 |
421±11 |
861±22 |
>5000 |
>5000 |
Lactam 7 |
>5000 |
>5000 |
536±32 |
96±25 |
>1000 |
>1000 |
Lactam 9 |
>5000 |
275±11 |
508±35 |
76±20 |
231±21 |
341±21 |
Lactam 19 |
>5000 |
76±20 |
>1000 |
326±29 |
31±2 |
38±6 |
Lactam 22 |
>5000 |
121±11 |
>1000 |
>1000 |
13±4 |
45±4 |
Lactam 24 |
>5000 |
231±21 |
>1000 |
>1000 |
46±7 |
72±8 |
Table 2 Cytotoxicity assay, IC50 of selected examples against a variety of breast, GI and lung cancer cell lines. IC50 values are based on inhibition of viability in the MTT assay
Xenograft in vivo lung cancer study
The fluorinated phenyl ethyl pyrrol 24 has had promising in vitro activity on cell lines in the nanomolar range. Lactam 24, went into preclinical development as PNB‒101 and was selected for further in vivo studies using nude mice and the results are outlined in Figure 5.
The transplanted lung tumour cell line proliferated exponentially and 100mg/kg reduced growths significantly (40% inhibition, day 24) and a further reduction was found dose dependently for the 200mg/kg dose and the antineoplastic effect appeared saturated for the 300mg/kg dose (64%, Day 24). Cis‒platin served as standard and killed the tumour, when treated early until resistance was observed on day 27. When cis‒platin was applied to an established tumour, no inhibitory effect on tumour growth was observed at all in cycle 2 for the group 4.
The acute toxicity was assessed as loss of body weight and cis‒platin showed a clear reduction of body weight, in contrast PNB‒101, which increased body weight at all doses, not significantly different from the control. No toxicity of this targeted experimental chemotherapeutic agent PNB‒101 was found and no signs of resistance as for cisplatin were observed for the entire period in the xenograft study. The importance of the gastrin receptor is not limited to lung cancer, but also linked with liver cancer and other gastrin related cancers, such as the cancer of the stomach. With PNB‒028 (cholecystokinin antagonist) and PNB‒101 (gastrin antagonist) targeted chemo‒selective agents will become available for a range of cancers, which were historically characterised by origin, and now, will become treatable based on a specific common endocrine growth factor.35
These novel pyrrol‒ones showed a good inhibition for CCK and gastrin related cancers. PNB‒101 was identified as best gastrin related anticancer agent and entered early preclinical development (PK analysis and membrane penetrations studies). Thus, 2 potent molecules PNB‒028 and PNB‒101 are available from fluoro‒benzene and furfural via the same stage 1 intermediate, making them readily available molecules with a great therapeutic potential. In vitro SAR evaluation has already indicated future therapeutic areas, in addition to lung cancer, such as triple resistant breast cancers, which are still an unmet therapeutic need. The known CNS activity of our CCK antagonists may be of additional therapeutic benefit in treating cancer associated diseases, such as pain, anxiety and depression.
The experimental work was partly supported by PNB Vesper Life Sciences.
Author declares that there is no conflict of interest.

©2018 Lattmann, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.