MOJ
eISSN: 2575-9094


Research Article Volume 1 Issue 2
1Institute of pharmaceutical chemistry, Saints Cyril and Methodius University of Skopje, Republic of Macedonia
2University Clinic of Cardiology, Republic of Macedonia
Correspondence: Ana Vavlukis, Faculty of Pharmacy, Saints Cyril and Methodius University of Skopje, Mother Theresa 17, 1000 Skopje, Republic of Macedonia, Tel 38 9070 3834 01
Received: April 01, 2017 | Published: May 26, 2017
Citation: Vavlukis A, Nestorovska AK, Sterjev Z, et al. Influence of ABCB1 C3435T genotype on clinical cardiovascular outcomes in coronary artery disease patients on Clopidogrel treatment. MOJ Drug Des Develop Ther. 2017;1(2):40-45. DOI: 10.15406/mojddt.2017.01.00007
Background: Clopidogrel has been used as a gold standard (alone or in association with aspirin) to prevent vascular complications in atherothrombotic patients undergoing PCI, and as a long-term prevention of cardiovascular events. The drug is subject to efflux via P-glycoprotein (encoded by the ABCB1 gen, also known as MDR1). The ABCB1 C3435T polymorphism may be associated with altered drug metabolism, efficacy and clinical outcome. The aim of this study was to evaluate the influence of ABCB1 C3435T genotype on clinical cardiovascular outcomes in coronary artery disease patients on clopidogrel treatment.
Methods: The study included a total of 203 subjects, of which 107 healthy volunteers and 96 patients diagnosed with coronary artery disease on treatment with clopidogrel from the Special Hospital for Surgical Diseases "Filip II". The genotyping of both the control group and the patient group was performed with Real-Time PCR based on the allelic discrimination method. Statistical analysis was performed using SPSS software. The genotype distributions were assessed for the Hardy-Weinberg equilibrium (HWE) with χ2 test.
Results: The C3435T allele and genotype frequency distribution testing in the patient group showed that the polymorphism is not associated with a risk of atherosclerotic cardiovascular disease (ACD) in the Macedonian population (p>0.05). A higher C-risk allele frequency was found in the subgroup of patients with negative outcome after treatment, compared to the subgroup of patients with positive outcome (0.5568 vs 0.4904; OR=1.306 T®C; p=0.35855). The same trend was observed when following the C3435T allele and genotype distribution in the subgroup of patients with normal CYP2C19 genetic status (CYP2C19*1/*1) (0.6400 vs 0.4868; OR=1.874 T®C; p=0.09105).
Conclusion: The results indicate that the ABCB1 C3435T polymorphism is an independent and complementary genetic factor that affects the outcome of clopidogrel treatment, but requires further research and a bigger study population.
Keywords: P-glycoprotein, Clopidogrel, ABCB1, pharmaco genetics, coronary artery disease, republic of macedonia
HWE, hardy-weinberg equilibrium; ACS, acute coronary syndromes; PCI, percutaneous coronary interventions; ACE, adverse cardiovascular events; CYP, cytochrome P450; ABC, adenosine triphosphate-binding cassette; MI, myocardial infarction; ST, stent thrombosis; CD, cardiac death; ACD, atherosclerotic cardiovascular disease
Platelet aggregation inhibition prevents the formation and progression of thrombotic events in patients presenting with acute coronary syndromes (ACS) and in those undergoing percutaneous coronary interventions (PCI) with stenting. The current treatment guidelines recommend dual anti platelet therapy with aspirin (in low doses) and the thienopyridine Clopidogrel.1 However, a relatively high incidence of inter individual variability in response to clopidogrel is reported. In addition, numerous observational studies demonstrate a correlation between reduced platelet response to clopidogrel and possible adverse cardiovascular events (ACE), including stent thrombosis.2
Clopidogrel, аn inactive pro drug, requires intestinal absorption by the P-glycoprotein, and in vivo bio activation by the cytochrome P450 (CYP) enzymes (including CYP2C19). Biotransformation generates its active thiol metabolite, which then targets the P2Y12 ADP-platelet receptor.3
The CYP2C19 genotype is the most important determinant of the pharmacokinetic and pharmaco dynamic response to clopidogrel, although it explains only 12-14% of the reported variability.4 The presence of any loss-of-function allele (*2, *3, *4, *5, *6, *7 and *8) results in enzyme deficiency, leading to reduced formation of the active metabolite, and thus, reduced anti platelet activity.5 Reduced CYP2C19 activity is associated with a risk of ischemic cardiovascular events in clopidogrel-treated patients, while increased enzyme activity determined by the CYP2C19*17 genetic variant may be associated with increased response to therapy, and thus, an increased risk of bleeding as an adverse event.6 To that end, the United States Food and Drug Administration has incorporated CYP2C19 genetic information-a black box warning into the clopidogrel label, indicating that CYP2C19 slow metabolizers may have a lack of therapeutic outcome, and recommended identificational genetic testing.7
P-glycoprotein (P-gp) is an adenosine triphosphate-binding cassette (ABC) efflux transporter encoded by the ABCB1 gene (also known as MDR1 gene - multidrug resistance protein 1 gene).8 Its main physiological function is the elimination of potentially toxic xenobiotics.9 It is expressed, among other places, on intestinal epithelial cells where increased expression or function can limit intestinal absorption, and thus, oral bioavailability of substrate drugs, such as Clopidogrel.10,11 The C3435T polymorphism has been associated with altered P-gp activity, the nature of the influence being highly inconsistent across different studies.8 Although some studies suggested an impact of the 3435TT genotype (mutant T allele being associated with low P-gp expression) on both clopidogrel responsiveness and risk of ACE; other studies reported a worse outcome in patients carrying the wild-type CC genotype.12–16 Thus, the aim of this study was to evaluate the influence of ABCB1 C3435T genotype on clinical cardiovascular outcomes in coronary artery disease patients on clopidogrel treatment.
Study population
The study included a total of 203 subjects, of which 107 unrelated healthy ethnical Macedonian volunteers of both sexes (76 males and 31 females), and 96 patients diagnosed with coronary artery disease on treatment with clopidogrel from the Special Hospital for Surgical Diseases "Filip II", Skopje, R. Macedonia. All patients were given a single high loading dose of 600 mg of clopidogrel, and continued with a maintenance dose of 75 mg daily for up to 15 months. The follow-up period was at least 12 months after treatment initiation for signs of a negative clinical outcome defined as MACE (major adverse cardiovascular events): myocardial infarction (MI), stent thrombosis (ST), stroke or cardiac death (CD). All procedures regarding subject selection and study research were approved by the ethics committee of the institution involved, and conducted in accordance with the Declaration of Helsinki.
DNA isolation
Genomic DNA was isolated from lymphocytes in peripheral blood samples obtained by venepunction in vacutainers with EDTA (Ethylenediaminetetracetic acid) as an anticoagulant. DNA extraction was done using a classical method of proteinase K digestion/phenol chloroform extraction and ethanol precipitation. DNA yields and purity were measured spectrophotometrically at 260 nm and 260/280 nm respectively (NanoDrop 2000c UV-Vis Spectrophotometer, Thermo Scientific, USA). DNA integrity was confirmed with electrophoresis on 1% agarose gels and visualization with fluorescent ethidium bromide. DNA samples were kept at 4˚C until further use.
Genotyping
Genotyping for ABCB1 C3435T polymorphism was performed with Real-Time PCR based on the allelic discrimination method. All reactions were performed on a fluorescent MxPRo 3005P thermal analizator (Agilent technologies, CA, USA) using specific TaqMan tests for SNP genotyping (TaqMan SNP Genotyping assay) of the C3435T SNP (rs1045642 assay ID C_7586657_20). Reaction settings were according to the guidelines of the manufacturer (Applied Bio systems, Life Technologies, and USA).
Statistical analysis
Statistical analysis was performed using SPSS software (v. 22). The genotype distributions were assessed for the Hardy-Weinberg equilibrium (HWE) with χ2 test using an online calculator (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). Chi-squared test and Fisher exact probability test were used for statistical analysis of allele and genotype frequency differences between the subgroups of patients with positive and negative outcome. Odds ratios (OR) were calculated with 95% confidence interval limits (95% CI). The level of statistical significant was defined as p≤0.05.
ABCB1 C3435T genotype distribution and allele frequency in healthy macedonian population
Distribution of the ABCB1 C3435T allele and genotype frequencies in healthy Macedonian population is presented in Table 1. The distribution was in agreement with the one predicted by the Hardy-Weinberg equilibrium. The wild-type allele frequency for C3435T was 51.4%, thus similar to the general frequencies reported for Caucasians of European descendant, but significantly different from those reported for Asian and African population. Allele frequencies in exon 26 for locus 3435 were 51.4% for the wild-type C allele and 48.6% for the mutant T allele, while the observed genotype frequencies were 25.2% for 3435CC, 52.3% for 3435CT and 22.5% for 3435TT.
SNP |
Locus |
Exon |
Allele |
N=214 Chromosomes |
Allele Frequencies (%) |
Genotype |
N=107 Healthy Subjects |
Frequencies (%) |
|
|---|---|---|---|---|---|---|---|---|---|
obs |
exp |
||||||||
rs.1045642 |
3435 |
26 |
C |
110 |
51.4 |
CC |
27 |
25.23 |
26.4 |
T |
104 |
48.6 |
CT |
56 |
52.34 |
49.9 |
|||
TT |
24 |
22.43 |
23.6 |
||||||
СС |
27 |
25.23 |
|||||||
СT+ТТ |
80 |
74.77 |
|||||||
Table 1 ABCB1 C3435T genotype distribution and allele frequency in healthy Macedonian population.
Notes: Distribution of the study subjects according to their ABCB1 C3435T genotype (CC, CT or TT) with respect to observed clinical outcome during the follow-up period.
ABCB1 C3435T genotype distribution and allele frequency in clopidogrel-treated patients
Distribution of the ABCB1 C3435T allele and genotype frequencies in the patient population is presented in Table 2. The distribution was in agreement with the one predicted by the Hardy-Weinberg equilibrium (P χ2=0.6701). Allele frequencies for locus 3435 were 52.08% for the wild-type C allele and 47.92% for the mutant T allele, while the observed genotype frequencies were 26.04% for 3435CC, 52.08% for 3435CT and 21.88% for 3435TT. No statistically significant difference was observed when comparing the allele and genotype distribution between the healthy volunteers and the patient population, thus the C3435T polymorphism was not associated with risk of atherosclerotic cardiovascular disease (ACD) in Macedonian population (p>0.05).
АBCB1 |
Patients (N=96) |
Control Group (N=107) |
||||
|---|---|---|---|---|---|---|
N |
Observed Frequency |
HWE Frequency |
N |
Observed Frequency |
HWE Frequency |
|
Genotype |
||||||
СС |
25 |
0.2604 |
0.2713 |
27 |
0.2523 |
0.2642 |
СТ |
50 |
0.5208 |
0.4991 |
56 |
0.5234 |
0.4996 |
ТТ |
21 |
0.2188 |
0.2296 |
24 |
0.2243 |
0.2362 |
Allele |
||||||
С |
100 |
0.5208 |
110 |
0.514 |
||
Т |
92 |
0.4792 |
104 |
0.486 |
||
Table 2 ABCB1 C3435T genotype distribution and allele frequency in clopidogrel-treated patients compared to healthy Macedonian population.
ABCB1 C3435T and clinical outcomes in clopidogrel-treated patients
Distribution of the ABCB1 C3435T allele and genotype frequencies in the two subgroups of patients followed for clopidogrel treatment outcome is presented in Table 3 and Figure 1. A total of 44 patients presented with a negative clinical outcome, defined as MACE, during or after clopidogrel treatment, compared with 52 patients with positive outcome. Although a higher C allele frequency was observed in the negative outcome subgroup, compared with the frequency in the positive outcome subgroup, it did not reach a statistical significance in the allelic and genotype model of statistical analysis (0.5568 vs 0.4904; OR=1.306 Т→C; p=0.35855). CC homozygotes were associated with an increased risk of MACE as compared with CT heterozygotes and TT homozygotes (OR=1.384 ТТ→CT, p=0.53993; OR=1.760 ТТ→CC, p=0.34565).
АBCB1 |
Patients with Positive Outcome (N=52) |
Patients with Negative Outcome (N=44) |
OR (95%CI) |
P (sig) |
||||
|---|---|---|---|---|---|---|---|---|
N |
Observed Frequency |
HWE Frequency |
N |
Observed Frequency |
HWE Frequency |
|||
Genotype |
||||||||
СС |
12 |
0.2308 |
0.2405 |
13 |
0.2955 |
0.31 |
1.76 |
0.34565 |
СТ |
27 |
0.5192 |
0.4998 |
23 |
0.5227 |
0.4935 |
1.384 |
0.53993 |
ТТ |
13 |
0.25 |
0.2597 |
8 |
0.1818 |
0.1964 |
1 |
|
Allele |
||||||||
С |
51 |
0.4904 |
49 |
0.5568 |
1.306 |
0.35855 |
||
Т |
53 |
0.5096 |
39 |
0.4432 |
1 |
|||
Table 3 ABCB1 C3435T allele and genotype frequencies in patients followed for clopidogrel treatment outcome.
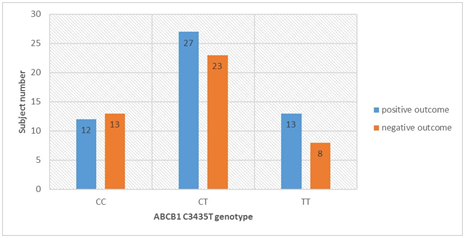
Figure 1 ABCB1 C3435T genotype distribution and clinical outcomes in clopidogrel-treated patients.
Notes: Distribution of the study subjects according to their ABCB1 C3435T genotype (CC, CT or TT) with respect to observed clinical outcome during the follow-up period.
ABCB1 C3435T and clinical outcomes in clopidogrel-treated patients with normal CYP2C19 (CYP2C19*1/*1) genetic status
Distribution of the ABCB1 C3435T allele and genotype frequencies in the two subgroups of patients with normal CYP2C19 (CYP2C19*1/*1) genetic status followed for clopidogrel treatment outcome is presented in Table 4 and Figure 2. Although a higher C allele frequency was observed in the negative outcome subgroup, compared with the frequency in the positive outcome subgroup, which did not reach statistical significance in the allelic and genotype model of statistical analysis (0.6400 vs 0.4868; OR=1.874 Т→C; p=0.09105), it was evident that the results showed a trend toward higher risk of negative outcome with clopidogrel treatment in ABCB1 C allele carrier patients. CC homozygotes were associated with an increased risk of MACE as compared with CT heterozygotes and TT homozygotes (OR=1.316 ТТ→CT, p=0.69829; OR=3.056 ТТ→CC, p=0.12665). Given the results before and after sub stratification according to CYP2C19 genetic status, ABCB1 C3435T arised as an independent predictor of adverse cardiovascular events.
АBCB1/Normal CYP2C19 Genotype |
Patients with Positive Outcome (N=38) |
Patients with Negative Outcome (N=25) |
OR (95%CI) |
P(sig) |
|||||
N |
Observed Frequency |
HWE Frequency |
N |
Observed Frequency |
HWE Frequency |
||||
Genotype |
|||||||||
СС |
9 |
0.2368 |
0.237 |
11 |
0.44 |
0.4096 |
3.056 |
0.12665 |
|
СТ |
19 |
0.5 |
0.4997 |
10 |
0.4 |
0.4608 |
1.316 |
0.69829 |
|
ТТ |
10 |
0.2632 |
0.2633 |
4 |
0.16 |
0.1296 |
1 |
||
Allele |
|||||||||
С |
37 |
0.4868 |
32 |
0.64 |
1.874 |
0.09105 |
|||
Т |
39 |
0.5132 |
18 |
0.36 |
1 |
||||
Table 4 ABCB1 C3435T allele and genotype frequencies in patients with normal CYP2C19 (CYP2C19*1/*1) genetic status followed for clopidogrel treatment outcome.
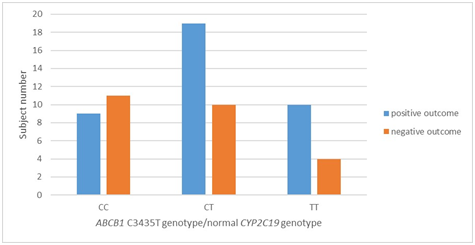
Figure 2 ABCB1 C3435T genotype distribution and clinical outcomes in clopidogrel-treated patients with normal CYP2C19 (CYP2C19*1/*1) genetic status.
Notes: Distribution of the subgroup of study subjects with normal CYP2C9 genotype (CYP2C19*1/*1) according to their ABCB1 C3435T genotype (CC, CT or TT) and observed clinical outcome during the follow-up period.
The exploration of novel genetic markers that may affect clopidogrel treatment response in patients with ACS and/or undergoing PCI with stenting has been the focus of numerous research projects in recent years. However, with an increasing number of results available, evidence on the association of ABCB1 C3435T genotype with clopidogrel clinical outcome has been contradictory.17
The present study aimed to determine whether the ABCB1 C3435T polymorphism was associated with an influence on clinical outcomes in clopidogrel-treated patients. It is evident that the results showed a trend towards higher risk of negative outcome with clopidogrel treatment in ABCB1 wild-type C allele carrier patients. Also, given the data before and after sub stratification according to CYP2C19 genetic status in the patient population, ABCB1 C3435T arised as an independent predictor of adverse cardiovascular events in patients with normal CYP2C19 genotype. We did not reach statistical significance in the allelic and genotype models of statistical analysis.
The ABCB1 gene encodes the efflux pump P-glycoprotein, also known as MDR1, a physiologic intestinal barrier against the absorption of several drugs, including Clopidogrel.11 The best studied mutation of the ABCB1 gene is a shift from C to T at position 3435, a genetic determinant of interethnic and inter individual variability in clopidogrel treatment response. The present study detected a mutant allele frequency for C3435T of 48.6% in the healthy ethnical Macedonian population, thus similar to the general frequencies reported for Caucasians of European descendant (46-56%), but significantly higher from those reported for the Asian (34-43%) and African (17-27%) population.18,19
Studies to date have been conflicting as to whether the non coding C3435T SNP affects the level of P-gp expression or activity, and as to which allele (genotype) is associated with increased/decreased P-gp expression.20,21 High expression of ABCB1 might therefore increase expression of the transporter and, thus, affect the systemic exposure to clopidogrel and its active metabolite. To that end, Taubert et al.3 investigated the effect of the variant allele on the circulating levels of clopidogrel and its active thiol metabolite. Patients homozygous for the 3435T allele had lower concentrations of the parent and the metabolite drug as compared to patients’ carriers of the 3435C allele, suggesting enhanced efflux possibly mediated by higher P-gp expression associated with the 3435TT genotype.1
Given the previously stated, one would expect an impaired platelet response and a negative clinical outcome as a consequence. Calderon-Cruz et al.13 demonstrated high on-treatment platelet reactivity as a response to clopidogrel among those patients undergoing PCI who were 3435TT homozygotes when compared to the CT/CC individuals.13 On the other hand, Tang et al.22 showed no statistically significant differences in the platelet response among different C3435T genotypes examining the impact of the variant on the pharmaco dynamic properties of clopidogrel in 670 patients after PCI.22
A meta-analysis of twelve studies (four involved platelet activity and ten involved clinical outcomes) indicated that the ABCB1 C3435T polymorphism might be a risk factor for early major adverse cardiovascular events (MACE) in patients on clopidogrel. The four platelet activity studies resulted in an association between high platelet activity and the ABCB1 C3435T polymorphism that was not statistically significant. The long-term MACE had no significant association with ABCB1 C3435T polymorphism. Three studies demonstrated that significant elevated risk of early MACE was associated with T allele, TT homozygote and CT+TT dominant genetic model. The ABCB1 C3435T polymorphism was unrelated to the rate of myocardial infarction, ischemic stroke and all-cause mortality. Seven cohort studies reported that stent thrombosis was not associated with ABCB1 C3435T polymorphism in all genotype models.14Regarding the clinical outcome of clopidogrel-treated patients alone, the FAST-MI investigators were the first to report an increased risk of MI, stroke or cardiovascular death in acute myocardial infarction patients carriers of the mutant 3435T allele (TT homozygotes as well as CT heterozygotes).15
The discrepancy of the results regarding the effect of the C3435T polymorphism on clinical cardiovascular outcomes in clopidogrel-treated patients is best shown by comparison of the two genetic sub studies of the TRITON-TIMI 38 and PLATO trials. On one hand, the TRITON-TIMI 38 genetic sub study showed that the 3435T allele was significantly associated with risk of the primary endpoint of cardiovascular death, MI or stroke and that TT homozygotes had a 72% increased risk of the primary endpoint as compared with CT/CC individuals.23 On the other hand, the PLATO genetic sub study demonstrated a higher rate of ischemic events and a higher risk of an negative treatment outcome in CC homozygotes as compared with CT/TT individuals.16 Despite biological viability of the PLATO genetic sub study results regarding the association of the wild-type C allele with an increased risk of adverse cardiovascular outcomes in CAD patients after clopidogrel treatment, which effect was in the same direction as the data of our present research, they are contrary to most of the currently available findings.
According to literature data, the CYP2C19*2 genetic variant explains up to 14% of the inter individual variability in clopidogrel treatment efficacy. The remaining variability is thought to be due to the impact of traditional risk factors (age, sex, dietary habits) and others, opposed to CYP2C19 less understood, genetic factors, such as the C3435T variation of the ABCB1 gene. Hence, in order to examine the independent effect of the polymorphism without concomitant influence of the CYP2C19 genetic variants, patients were sub stratificated by their CYP2C19 genetic status, and only patients with normal CYP2C19 genetic status were analyzed (CYP2C19*1/*1). The allele and genotype frequency distributions remained the same before and after the sub classification. A higher C allele frequency was observed in the negative outcome subgroup, compared with the positive outcome subgroup, and an increased risk of an adverse cardiovascular event (ACE) was seen in the patient population homozygous for the wild-type allele (CC homozygotes), compared with the CT/TT individuals. Despite not reaching statistical significance, the results showed a trend toward higher risk of ACE in ABCB1 C allele carrier patients. The obtained data can be explained with the scientific speculation that ABCB1 C3435T may be a significant, independent predictor of adverse cardiovascular events-myocardial infarction, stroke, stent thrombosis and cardiovascular death in patients with normal CYP2C19 genotype.
In recent years efforts are being made towards establishing a clinical practice based on individual patient approaches. That means that accent is put on the experience and clinical judgment of the physicians to choose an appropriate anti platelet drug by taking into account the benefits of the treatment on one side, and the risk of ischemic and bleeding events on the other. Contrast opinions still exist as to which method, phenol typing, genotyping, or a combination of both, is the most useful in everyday clinical settings. And if this was not enough, there is still controversy as to which genetic markers have a clinically relevant influent on clopidogrel treatment efficacy to be included in the genotyping future. Thus, further studies are much needed to develop the principle of individualized anti platelet therapy taking into account the patient genetic background.
Study limitations
This study is the first to be evaluating the influence of the ABCB1 C3435T polymorphism on clinical cardiovascular outcomes in clopidogrel-treated patients in Macedonian population. It has several limitations that are worth mentioning. We only assessed the impact of C3435T SNP of the ABCB1 gene on clopidogrel clinical outcomes, not taking into account other genetic markers such as CYP2C19*3, CYP2C19*17, CYP2C9*3 and PON1 Q192R proven to affect clopidogrels’ pharmacokinetics, pharmaco dynamics and clinical outcome. We did not analyze plasma levels of clopidogrel, or its active metabolite, and we did not measure platelet aggregation, which would have given mechanistic insights into the data observed. One big limitation of our study is the small sample size, especially of the patient population, which could not lead to statistical significance of the results. With some statistical decision-making during the results evaluation, we saw that statistical significance could be reached by doubling the patient population, especially the studied negative outcome patient group. In other words, a negative outcome subgroup of about 100 patients, given the same trend in the positive outcome subgroup, could be representative enough to draw a statistically significant conclusion. Finally, 100% of the study cohort was white, so these results might be relevant only to white population. Additional studies that include a larger study population will be particularly important.
In coronary artery disease patients on clopidogrel treatment, the concomitant presence of CYP2C19*2 allele and one or two wild-type C allele/s (CC homozygotes and CT heterozygotes) has no additive effect on the risk of adverse cardiovascular events. Carriers of the CYP2C19*2 allele do not require ABCB1 C3435T genotyping. The need for ABCB1 C3435T genotyping is not excluded in patients with extensive CYP2C19 phenotype, giving the fact that ABCB1 C3435T could be an independent predictor of adverse cardiovascular events-myocardial infarction, stroke, stent thrombosis and cardiovascular death.
None.
The author declares no conflict of interest.

©2017 Vavlukis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.