MOJ
eISSN: 2575-9094


Research Article Volume 2 Issue 4
1Department of Pharmacy, Kohat University of Science and Technology, Pakistan
2Department of Pharmacy, University of Peshawar, Pakistan
3Department of Pharmacy, Sarhad University of Science and Technology, Pakistan
Correspondence: Zafar Iqbal, Department of Pharmacy, University of Peshawar, Pakistan, Tel +92-91-9239619
Received: July 28, 2018 | Published: August 9, 2018
Citation: Khan A, Iqbal Z, Niaz N. Evaluation of the effect of co–grinding on dissolution rate of poor water soluble drug (clarithromycin). MOJ Drug Des Develop Ther. 2018;2(4):232-237. DOI: 10.15406/mojddt.2018.02.00052
Most of the newly invented active pharmaceutical ingredients (APIs) are poor water soluble and dissolution rate is the limiting step in bioavailability from compressed solid dosage forms. Objective of the study was to elucidate the effect of hydrophilic carriers on dissolution rate of poor water soluble drug and utilization of co‒grinded Clarithromycin in preparation of tablets by direct compression. Intrinsic dissolution rate of Clarithromycin was determined according to United States Pharmacopeia (USP) using rotating disk method. Hydrophilic carrier (micro crystalline cellulose) was applied in varying ratios (1:1, 1:2 and 1:4; drug to carrier ratio by weight) for enhancement of dissolution rate of Clarithromycin by co‒grinding technique. Co‒grinded Clarithromycin was prepared by compressing lubricated physical mixture (blend of Clarithromycin and micro crystalline cellulose) to slugs, followed by granulation. Tablets containing co‒grinded Clarithromycin were prepared by direct compression technique and evaluated for various official and unofficial parameters at pre compression and post compression level. The prepared tablets were compared with marketed tablets in terms of physical parameters, mechanical strength, disintegration behavior and in‒vitro drug release. Dissolution profiles of both types of the tablets were compared on the basis of maximum drug release, dissimilarity factor (f1), similarity factor (f2) and dissolution efficiency. Clarithromycin is a class‒II drug, with poor water solubility. Its intrinsic dissolution rate is very low (0.678mg/cm2.min). In comparison to intrinsic dissolution rate, significant increase in dissolution rate of Clarithromycin was observed by co‒grinding with hydrophilic carriers (267.11%). Dissolution rate of Clarithromycin enhanced with concentration of hydrophilic carrier and maximum increase was observed at 1:2 (drug to carrier ratio by weight). By further increasing ratio of the carrier resulted in decrease in dissolution rate due to cushioning effect. Tablets containing co‒grinded Clarithromycin, showed better dissolution profile compared with marketed tablets. In comparison with marketed tablets, maximum drug release in 60min, Q60 (99.73±0.39), Q30 (75.81±0.19%), Q15 (31.17±0.26%), and dissolution efficiency (93.73%) were higher for tablets containing co‒grinded Clarithromycin. Dissolution rate of poor water soluble drug (Clarithromycin) can be enhanced by co‒grinding with hydrophilic carrier (micro crystalline cellulose). Co‒grinding is an environment friendly technique that can be applied for enhancement of dissolution rate of poor water soluble APIs irrespective of nature and dose.
Keywords: clarithromycin, co‒grinding, direct compression
In order to produce its therapeutic effect, the drug must be released from the dosage form and reach the general circulation. Aqueous solubility and permeability of any active Pharmaceutical ingredient are the key properties as these govern dissolution rate and subsequent absorption from gastro intestinal tract.1 Most of the newly invented APIs are hydrophobic in nature having poor water solubility and good permeability i.e. when released from the dosage form is rapidly absorbed. Dissolution rate is the limiting step in oral bioavailability of hydrophobic drug. To improve their dissolution rate and bioavailability, various techniques have been employed such as particle size reduction,2 complexation with hydrophilic moieties,3 preparation of solid dispersions,4 liqui‒solid technique,5 salt formation,6 changes in physical form, pro drug approach, drug derivatization, alteration of micro environmental pH and addition of surfactants. Solid‒dispersion technique has been extensively applied for enhancement of dissolution rate of hydrophobic APIs with a wide range of solubility.6,7 Solid‒dispersion technique has been proved to be the most successful in improving the dissolution rate because the drug interacts with hydrophilic polymer at molecular level, resulting in enhanced drug water interaction and subsequently increased solubility and dissolution rate. Hydrophilic polymers with a wide range of physico‒chemical characteristics are available which can change characteristics of the APIs, dramatically.8,9 Despite its promising results, a lot of problems are associated with practical applicability of the solid dispersion technique, especially at the commercial level. The process is associated with various manufacturing problems like excessive use of organic solvents, dose limitations,10 poor physical characteristics of finished product and difficulty to be scaled up for commercial applications.11,12 There is need for a technique of improving dissolution rate that is solvent free, can be applied for large dose of hydrophobic drugs and can be scaled up for commercial applications. Physical modifications have been the focus of research during last decade and deals with increase in surface area of API leading to higher solid‒liquid interactions.13 Particle size reduction is mainly achieved by milling, applying a varying degree of techniques and equipments. Particle size reduction is losing its importance at commercial level due to sensitive nature of APIs (heat sensitive), loss during processing and time consumption. Co‒grinding with hydrophilic carriers enhances dissolution rate due to particle size reduction and drug carrier interaction at micro level. Co‒grinding affects physical properties of APIs like specific area, crystalline structure and shape, contributing to interactions with dissolution media. It is a solvent free technique and is considered to be environment friendly which can be applied for a number of drugs, irrespective of the dose and solubility.14 It is an easy, cost effective and fast process involving simple equipments that can be easily scaled up for commercial applications.
The available literature about co‒grinding as dissolution rate enhancement process is limited despite the fact that a variety of hydrophilic polymers are available which can dramatically change dissolution behavior of the APIs. Co‒grinding can be considered as an alternative for conventional solid dispersion as the drug and polymer has close interaction, leading to enhanced wettability of the drug. In the present study, Clarithromycin was selected as model drug due to its poor water solubility. Clarithromycin is a semi‒synthetic macrolides antibiotic. Chemically, it is 6‒Omethylerythromycin. The molecular formula is C38H69NO13. Molecular weight is 747.96.15 Clarithromycin is a white to off‒white crystalline powder. It is soluble in acetone, slightly soluble in methanol, ethanol and acetonitrile and practically insoluble in water.16 Objectives of the study are to enhance dissolution rate of poor water soluble drug (clarithromycin) by co‒grinding with hydrophilic carrier (microcrystalline cellulose). In the present study, cogrinding of clarithromycin was carried out by slugging and granulation technique, using micro crystalline cellulose as hydrophilic carrier in different ratios. Cogrinded formulation with maximum dissolution rate was used for preapartion of tablets by direct compression. Tablets containing cogrineded clarithromycin were compared with marketed tablets in terms of various official and un‒officila parameters.
Material and equipments
Model drug clarithromycin (purity 99.72% in comparison to USP standard) was gifted by Ferozsons Laboratories Pvt. Ltd. Nowshera, Pakistan. Rests of the excipients (micro crystalline cellulose, tablettose‒80, magnesium stearate, and primojel) were purchased from local market of Peshawar. All the materials were of pharmaceutical grade and were used as received. Digital balance (Precisa, Switzer Land), laboratory scale double cone mixer (Morgan Instruments, Pakistan), Rotary granulator (STC, China), rotary compression machine (ZP‒21, China) and dissolution testing apparatus (Pharma Test, Germany) were used during preparation and evaluation of co‒grinded formulations.
Determination of solubility of clarithromycin
Solubility of the clarithromycin was determined by dissolving weighed quantity of powder in sufficient amount of purified water (pH5–7) at ambient temperature, using standardized shake flask method. Drug (1g) was weighed accurately using electronic balance and transferred to a volumetric flask (capacity=100mL) containing purified water (50mL). The flask was shaken for 45min using mechanical flask shaker (Morgan Instruments, Pakistan), noted solubility state of the drug and repeated the procedure after further addition of the drug. The process was repeated till complete saturation of the solution with the drug. Solubility of clarithromycin was calculated using following Equation 1.
Solubility= (Amountofclarithromycin (mg))/(Volumeofsolution (mL))Co‒grinding of clarithromycin with hydrophilic excipient
Slugging technique of cogrinding comprised of preparation of powder blend, compression of powder blend to slugs and granulation of slugs. For preparation of physical mixture, clarithromycin and hydrophilic excipients (micro crystalline cellulose) were weighed accurately using electronic balance (Precisa, Switzerland), in different drug to excienpt ratio (1:1, 1:2, and 1:3 by weight). All the ingredients of the mixture were sifted through mesh number 30 and blended in a laboratory scale double cone blender (Morgan Instruments, Pakistan) for 15min. at 25rpm. Mixture of drug and micro crystalline cellulose was lubricated with magnesium stearate (1% w/w). Lubricated powder blend was compressed to slugs using rotary compression machine (ZP‒21, China). Round flat punches (15mm) were used for slugging of powder blend under compression force of 40–50kN. Slugs were granulated through mesh number 40 using rotary granulator (STC, China), resulting in cogrinded clarithromycin. Figure 1 shows schematic presentation of the process of preparation for cogrinded clarithromycin.
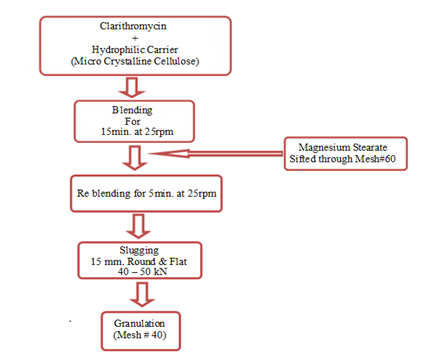
Figure 1 Schematic presentation of the process of co‒grinding of clarithromycin with micro crystalline cellulose.
Drug release studies of the cogrinded clarithromycin
Drug release studies of the cogrinded clarithromycin were performed by the method described for determination of intrinsic dissolution rate, using rotating disk method at 100rpm.17 Dissolution media consisted of 0.1N HCl (900mL) held at 37±2oC. Sample (5mL) was withdrawn at specified time intervals (0, 5, 15, 30, 45 and 60min), filtered and analyzed for amount of drug released using UV visible spectrophotometer (Shimadzu, Japan). After each sampling, volume was corrected with same volume of dissolution media, held the same temperature.
Preparation of tablets containing co‒grinded clarithromycin
Direct compression technique was applied for preparation of tablets containing co‒grinded clarithromycin. Maximum dissolution rate was used as criteria for selection of co‒grinded clarithromycin for preparation of tablets. Maximum dissolution rate was observed with micro crystalline cellulose (1:2, drug to carrier ratio by weight). Co‒grinded clarithromycin was blended for 15min, with rest of the excipients as per Table1, using laboratory scale double cone mixer. Powder blend was compressed using rotary compression machine (DH‒29, Yanchen, Taiwan) fitted with 19mm oval punches, with bisection line. Compression weight of the tablets was 900mg/tablet and at least 500tablets were compressed.
Ingredient |
Quantity |
|
|---|---|---|
% (w/w) |
mg/tablet |
|
Co‒grinded Clarithrocycin* |
83.3 |
750 |
Tablettose‒80 |
12.7 |
114 |
Cross carmellose sodium |
3 |
27 |
Magnesium Stearate |
1 |
9 |
Total |
100 |
900 |
Table 1 Composition of the tablets containing co‒grinded clarithromycin
*Co‒grinded clarithromycin having highest dissolution rate was selected for tablet preparation
Comparison of tablets containing co‒grinded clarithromycin and marketed tablets
Tablets containing co‒grinded clarithromycin were compared with conventional tablets the same strength, selected on the basis of best annual sail report in Peshawar. Both types of the tablets were compared in terms of physical parameters, mechanical strength, disintegration behavior and in‒vitro drug release. All the parameters were determined for both types of the tablets, by the methods described in USP and compared. As objective of the co‒grinding was to enhance the dissolution rate so special emphasis was given to the comparison of dissolution profile of two types of the tablets.
Determination of drug content of tablets
Sample was prepared by finely crushing 10tablets and taking the quantity of powder equivalent to 250 of Clarithromycin in 100mL flask, containing methanol (65mL) and shaken for 20minutes and made the volume up to 100mL with methanol. After filtration through Wattman filter paper 1mL was diluted with 5mL of Dimethyl amino benzeldehyde solution (0.5% in Acetic acid) and 20mL acetic acid and volume made up to 50mL with mixture of acetic acid and HCL (35:70) and the solution was kept in dark for 15minutes. Standard solution was prepared by accurately weighing and dissolving 250mg Clarithromycin working standard in 100mL volumetric flask containing 65mL methanol and shaking done to dissolve and methanol was added to make volume 100mL. 1mL solution from the 100mL was taken in 50mL flask and 5mL Dimethyl amino benzaldehyde solution (0.5% in acetic acid) and 20mL of acetic acid added and then volume made up to 50mL with mixture of acetic acid and HCL (35:70). Final solution was kept in dark for 15minutes like the sample one and the absorbance measured for both sample and standard solutions at 485nm while using mixture of acetic acid and HCL (35:70) as a blank. Percentage of clarithromycin was calculated by Equation 2.
%Drug content = A sample / (A Standard)× 100In‒vitro drug release from tablets
Dissolution rate of clarithromycin was determined according to USP using apparatus‒1 at 100rpm.17 Dissolution media consisted of 0.1N HCl (900mL) held at 37±2oC. Sample (5mL) was withdrawn at specified time intervals (0, 5, 15, 30, 45 and 60min), filtered and analyzed for amount of drug released, using UV visible spectrophotometer (Shimadzu, Japan).
Comparison of In‒vitro drug release profile of marketed tablets and tablets containing co‒grinded clarithromycin
Model independent approach was applied for comparison of dissolution profile of marketed tablets and tablets containing co‒grinded clarithromycin. Similarity factor (f2), dissimilarity factor (f1) and dissolution efficiency (D.E.) were determined from dissolution profiles of the two types of tablets and compared. Dissimilarity factor (f1) was calculated using the Equation3 and similarity factor (f2) was calculated using Equation 4.18,19
f1=∑[Rt−Tt]∑Rt× 100 (Eq. 3)
f2= 50 × log {[1 + (1/n) ∑(Rt− Tt)2]−0.5× 100}(Eq.4)
Where
Rt = Dissolution rate of standard product at time “t”
Tt = Dissolution rate of test product at time “t”
The “f2” value of 50 or greater ensures sameness or equivalence of the two dissolution profiles and performance of the two products. Dissolution efficiency is the percentage of the area of rectangle described by 100% dissolution.19 It was calculated according to the following Equation 5.
D.E. = yxdtY100xT× 100(Eq.5)
Where
DE=Dissolution efficiency
y=Amount of drug released in time “t”
Y100=100 percent drug released
T=Total time
Dissolution efficiency was calculated for the marketed tablets and tablets containing co‒grinded clarithromycine at 30min and 60min.
Determination of solubility of claritromycin
According to biopharmaceutical classification system, clarithromycin is a class‒II drug and is practically insoluble in water.15 In comparison with reported water solubility, clarithromycin was practically insoluble in water; however increase in temperature enhanced its solubility which was comparable to its reported water solubility.
Determination of intrinsic dissolution rate of clarithromycin
Intrinsic dissolution rate is the dissolution rate of pure active pharmaceutical ingredient when conditions such as surface area, temperature, agitation or stirring speed, pH, and ionic strength of the dissolution medium are kept constant.17 Intrinsic dissolution rate was determined according to USP, using rotating disk method in dissolution media prescribed for determination of dissolution rate of clarithromycin from compressed tablets. For the accuracy of the results, intrinsic dissolution rate was determined at three different points and mean value was taken. Furthermore, at each sampling point analysis was carried out in triplicate (n=3). Intrinsic dissolution rate of clarithromycin was very low (0.678mg/cm2min.) as shown in Figure 2. Intrinsic dissolution rate depends upon physicochemical characteristics of the drug like solubility and crystalline structure and experimental conditions (compaction pressure and agitation rate). During the study the compaction pressure was optimized on the basis of compatibility of the disk. Low compression pressure will result in a disintegrating disk leading to positive deviation in the result due to increase in surface area. Higher compaction pressure will cause the drug particles to be tightly bound and unable to release leading to very low dissolution rate. The compaction pressure in the range of 70–90N was sufficient to prevent disintegration of the disk, without affecting drug release.
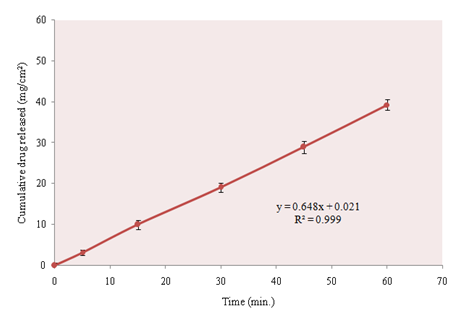
Figure 2 Intrinsic dissolution rate of clarithromycin, determined according to USP/NF by rotating disk method. dissolution media consisted of 0.1N HCl and speed of disk rotation was 100rpm.
Dissolution rate clarithromycin from co‒grinded formulations
Co‒grinded formulations exhibited remarkably enhanced dissolution rate than the intrinsic dissolution rate of clarithromycin. Hydrophilic excipient (micro crystalline cellulose) enhanced dissolution rate due to its solubalizing effect and improving wet ability by forming a physical complex, reduced particle size and changes in crystalline structure. Micro crystalline cellulose is a hydrophilic polymer, commonly used as diluents in formulation of solid dosage form. It also has good disintegrating properties and is considered one of the safe pharmaceutical excipients.20 Increase in dissolution rate of clarithromycin from co‒grinded formulations can be explained on the basis of various mechanisms as;
At lower drug to carrier ratio (1:1) dissolution rate was increased up to 198.52% of intrinsic dissolution rate as shown in Figure 3. Increase in the ratio of the carrier further enhanced the dissolution rate. Maximum dissolution rate was observed at 1:2 and was 267.11% of the intrinsic dissolution rate. Further increase in the ratio of the carrier, did not enhance the dissolution rate, significantly. At 1:4 (drug to excipient ratio) dissolution rate was smaller than that at 1:2. It may be due to the cushioning effect of the excipient. The drug particles were surrounded by the carrier particles and not exposed to attrition completely. To get the better results with co‒grinding, ratio of the drug to the hydrophilic carrier should be optimized to achieve maximum hydration and micronization of drug particles without cushioning effect.
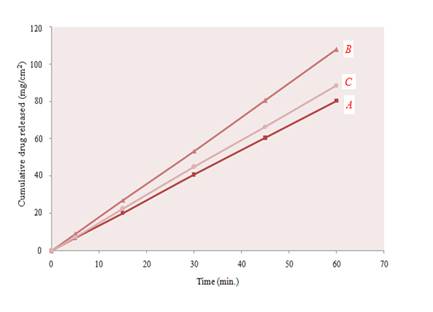
Figure 3 Dissolution rate of clarithromycin from co‒grinded formulations with MCC, prepared by slugging technique in different ratios (A; 1:1, B; 1:2, C; 1:4).
Post compression evaluation of the tablets containing co‒grinded clarithromycin and comparison with marketed tablets
A highly selling brand of clarithromycin was selected as standard tablets. Physical parameters (tablet dimension and compression weight) of the test tablets were kept similar to the marketed tablets i.e. the tablets were compressed using similar shaped punches under same compression weight. After compression, test tablets were evaluated for various official and unofficial parameters and compared with marketed tablets.
Physical characteristics of marketed tablets and tablets containing co‒grinded clarithromycin
Marketed tablets of clarithromycin were oblong shallow convex with 19mm diameter with bisection line on one side and “f” on the other. Compression weight of the tablet was 900mg/tablet. Similar compression tools were used for preparation of tablets from test formulation under the same compression weight (900mg/tablet) so that both the tablets have same dimensions. The theoretical weight of the tablets containing co‒grinded clarithromycin was 900mg, having weight variation within the official limits. Lower value of weight variation indicates proper flow of the powder during compression. Tablets from the test formulation were smooth and shiny without any sticking. Moisture content of the tablets was below 3% Table 2. The optimum moisture content is important for good mechanical strength and friability of the tablets and edging and capping were not observed in tablets from test formulation. Drug content of the tablets was above 99% which was within the official limits. Uniform drug content confirmed proper mixing of the drug with excipients.
Characteristics |
Test formulation |
Marketed tablets |
Weight Variation (%) |
±3.91 |
±3.27 |
Tablet Thickness (mm) |
5.28±0.34 |
5.53±0.21 |
Moisture Content (%) |
2.19±0.28 |
2.69±0.83 |
Drug Content (%) |
99.91±0.51 |
99.89±0.99 |
Table 2 Physical parameters of marketed tablets and test formulation (tablets containing co‒grinded clarithromycin)
Results are presented as Mean±S.D.
Mechanical strength of the tablets
Mechanical strength of the tablets was determined on the basis of crushing strength, specific crushing strength, tensile strength and tablet friability. All these parameters were determined according to the official compendia (USP), for both marketed tablets and tablets from test formulation. All the parameters related to mechanical strength were higher for the tablets containing co‒grinded clarithromycin than the marketed tablets, as shown in Table 3. Results of the mechanical strength of the tablets indicated that co‒grinded has no adverse effect on strength of the tablets.
Characteristics |
Test formulation |
Marketed tablets |
Crushing strength (Kg)† |
14.37± 0.86 |
14.61±0.93 |
Specific crushing strength (kg/mm2 )* |
‒ |
‒ |
Tensile strength (kg/mm2 )* |
‒ |
‒ |
Friability (%) |
0.15 |
0.31 |
Table 3 Comparison of mechanical strength of marketed tablets test formulations (tablets containing co‒grinded clarithromycin)
†: Results are presented as Mean±Standard Deviation (n=10)
*: Calculated on the basis of mean crushing strength and mean thickness of tablets
Disintegration behavior of the tablets
Disintegration time was used as indicator of the disintegration time of the tablets and was determined according to the official compendia (USP). Disintegration time of both types of the tablets was within the official limits (≤15min). Comparison of disintegration time of commercial tablets (8.25±0.19min) and tablets from test formulation (4.50±0.33min) Figure 4 showed that tablets containing co‒grinded clarithromycin exhibited rapid disintegration, despite their higher mechanical strength.
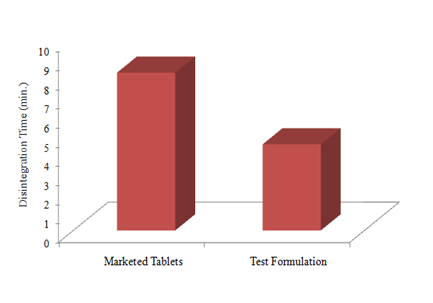
Figure 4 Comparison of disintegration time of commercial tablets of clarithromycin and tablets containing co‒grinded clarithromycin. dissintegration time was determined in purified water at 37±2oC for six tablets (n=6) and mean value was taken.
Comparison of dissolution profile of marketed tablets and tablets containing co‒grinded clarithromycin
Tablets were prepared by direct compression, using co‒grinded formulation of clarithromycin with MCC in 1:2 (drug to carrier ratio by weight) and cross carmellose sodium (cross linked carboxy methyl cellulose sodium) as disintegrant. Dissolution rate was determined according to USP using 0.1N HCl (900mL) as dissolution media and compared with that of commercially available tablets. In‒vitro drug release from tablets containing co‒grinded formulation was significantly high both in terms of maximum drug released and T50% Figure 5. Similarity factor and dissimilarity factors were calculated for dissolution profile of both types of tablets indicating no similarity between the two dissolution profiles. With co‒grinded formulation maximum drug release was 99.73±0.39% (n=6) which was 28.74% higher than the commercial tablets. Moreover dissolution efficiency, Q15min and Q30min were also higher for tablets containing co‒grinded clarithromycin Table 4, indicating better dissolution profile. Increase in wetability and decrease in particle size resulted in better dissolution profile of the tablets containing co‒grinded clarithromycin.
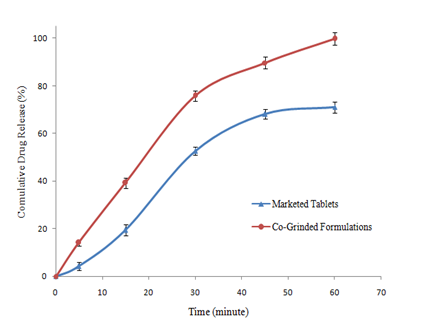
Figure 5 Comparison of dissolution profile of commercial tablets of clarithromycin and tablets containing co‒grinded clarithromycin prepared by direct compression. dissolution rate was determined in 0.1nhcl (900ml) at 37±2oC.
Parameter (unit) |
Marketed tablets |
Co‒grinded formulations |
Maximum drug released (%) |
71.07±0.18 |
99.73±0.39 |
Dissolution efficiency (%) |
71.07 |
93.73 |
Q15min (%) |
19.46±0.31 |
31.17±0.26 |
Q30min (%) |
52.71±0.47 |
75.81±0.19 |
Table 4 Comparison of dissolution profiles of marketed tablets and tablets containing co‒grinded clarithromycin
Results are presented as mean±standard deviation (n=6)
Aim of the study was to enhance the dissolution rate of class‒II drug (clarithromycin) by co‒grinding with hydrophilic carrier (micro crystalline cellulose) and its application in preparation of tablets by direct compression technology. Dissolution rate of clarithromycin was significantly increased by co‒grinding with hydrophilic excipient (microcrystalline cellulose). Co‒grinding with hydrophilic excipient is cost effective and environment friendly technique that can be applied for large doses of poor water‒soluble drugs at commercial scale without any problem of scaling up. Co‒grinding reduces the particle size of drug, changes crystalline structure and increases amorphousness; leading to enhanced dissolution rate. Increase in dissolution rate by co‒grinding with hydrophilic carriers is governed by the nature and quantity of hydrophilic carrier. Tablets containing co‒grinded clarithromycin exhibited better results for various official and un‒official tests.
None.
The author declares that there is no conflict of interest.

©2018 Khan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.