MOJ
eISSN: 2575-9094


Research Article Volume 2 Issue 4
Laboratory of Biotechnology Pharmaceutical, Institute of Biotechnology Pharmaceutical and Food-CONICET-UNT, Argentina
Correspondence: Maria Eugenia Sesto Cabral, Laboratory of Biotechnology Pharmaceutical, Institute of Biotechnology Pharmaceutical and Food-CONICET-UNT, Argentina, Tel +54-3814856596
Received: July 31, 2018 | Published: August 6, 2018
Citation: Eugenia M, Moreno, Julieta M, et al. Closing the life cycle of the pharmaceutical ingredients from biological origin a green interface to waste management. MOJ Drug Des Develop Ther. 2018;2(4):227–230. DOI: 10.15406/mojddt.2018.02.00051
One of the greatest advantages of pharmaceutical biotechnology is the versatility. And although its approach to the production of drugs opens a plethora of green and sustainable possibilities, the generation of waste continues to represent a problem on a smaller scale, but it can be brought to near zero. This work proposes an alternative for recovery, treatment and reintroduction of waste in the production lines. Through the recovery of bacterial biomass residues, generated during the production of Active Pharmaceuticals Ingredients and excipients, we were able to extract concentrates of protein useful for the design of new culture media. We present 12 media designed with the processed waste, which have the same or better production yield than the commercial reference medium. In all cases, biomass production exceeds that obtained when growing under the same conditions in commercial media. This approach allowed us to solve a waste management problem, to reduce costs and to generate high added value products from waste. Its degree of innovation allowed us to protect the methods and prototypes in a patent application.
Keywords: pharmaceutical biotechnology, clean production, culture media design
Significant advantage over chemical synthesis is that biological ones takes less production time but can often use more expensive raw materials. Cost, is one of the main factors in economics of biotechnological production. Refined sugars and protein sources, although costly, are the most commonly used substrates for commercial production of metabolites by fermentation process.1 The trend towards environmental sustainability and development of renewable resources has significantly increased interest in the recovery of fermentation products, organic acids, and industrial chemicals. A range of products obtained by fermentation is expanding beyond the traditional high‒volume and low‒value compounds, like pharmaceuticals, competing with traditional synthetic production of commodity chemicals. As fermentation moves into higher values and low‒volume chemicals, it becomes necessary to maximize efficiency and minimize costs and wastes to compete effectively against traditional options. Researchers and I+D+i teams from biotechnological industries must think about the end of the life cycle, before they create products. Our research group develops bacterial products for pharmaceutical industries, such as active pharmaceutical ingredients (API) and excipients also known as non‒active pharmaceutical ingredients (NAPI). Our scaling up process was developed by thinking beyond end‒of‒pipe solutions by treating the problem of waste generation since the beginning of the research development. In a previous work we define our own critical process points.2 By replacing sugar and protein sources it was possible to maintain or increase the efficiency of production process. By reentering our own wastes in our process line, we manage to replace expensive constituents, maintaining LAPS antipathogenic properties.3 This paper presents our zero waste protocol, designed to reuse remnant biomass, transforming it into high added value products. In this manner, we use three critical waste production points and bacterial residual cells (BRC) which were treated by physical techniques for its content release. Three lines and bacteria from our research program were selected for this purpose, all these processes generate BRC. Bacteria are Pseudomonas fluorecens wild type used for nano cellulose production, Lactobacillus alimentarius wild type and Enterococcus faecium wild type, used for exopolysaccharides obtention. The three of them were used as microbial cell factories.3,4 The main objective in this work was to design a non‒waste process production line, by transforming remnant biomass in higher values and low volume products. In addition, we expect to reintroduce the products obtained in the production lines as substitutes for the commercial protein sources, in order to create a green interface and to reduce costs.
Bacterial strains and culture conditions
Two strains of lactic acid bacteria were grown in Man Rogosa Sharpe (MRS‒Britania‒CABA‒Argentina) broth and kept at 37°C: Lactobacillus alimentarius wild type and Enterococcus faecium wild type. A strain of Pseudomonas fluorecens wild type was grown in LB broth (Britania‒C.A.B.A‒Argentina) and kept at 25ºC.
Residual Biomass reconversion
Bacterial residual cells (BRC) isolated from all process were washed with saline solution and then centrifuged at 8000rpm. for 10min (Arcano, 80‒2b).
Lysis process
BRC pellet was previously submitted to 4 lysis methods. High pressure and high temperature method, with water, at 121°C for 15min, was the chosen one, for its higher proteins release concentration.
Chemical characterization
For protein concentration assays using UV method were performed. The calibration curve was made by using bovine albumin as positive control and pH determination (pH Lutron).
Physical characterization
Dry pellet were obtained by placing BRC on a stove at 80ºC for 12h. Physical characterizations were made from dry pellets: dry weight, humidity % and residual humidity (data no shown).
Culture media design
In order to obtain high value products, which also allow us to reduce the cost of production, 16 culture media were designed, by replacing 50% and 100% of proteins from commercial media. Table 1 shows composition of all media designed. When L. alimentarius residual cells were used, 4 media were designed and named BCM1 (Blessing Cabrera Moreno) to BCM4. When E. faecium residual cells were used, 5 media were designed and named CaGB1 (Cabrera Gonzalez Blessing) to CaGB5.When P. fluorecens residual cells were used, 3 media were designed and named GS1 (Gonzalez Sesto) to GS3.
Bacterial growth in designed media
In order to define applicability of all new media, we test growth kinetics by using MRS and LB media respectively as positive control. Media designed (BCM1 to BCM4, CaGB1 to CaGB5) were used as broth for Lactic Acid Bacteria (LAB) static cultivation. Lactic acid bacteria were grown for 12h at 37ºC in MRS media, then suspensions of OD600≈0.150 were prepared using fresh medium as diluent and blank. P. fluorecens was grown for 24h at 25ºC in LB media, then suspensions of OD600≈0.150 were prepared using fresh medium as diluent and blank. GS1 to GS3 media were tested as broth for static cultivation. In 96‒well polystyrene microtiter plates (Costar Corning Inc. USA), 200μL of each media designed and controls were placed, and then 20μL of bacterial suspension were added. The plates were incubated for 12h at 37°C and growth curves were obtained by measuring the OD600 once per hour in a microplate reader (Thermo Scientific Multi scan Go). Each curve was performed by quintuplicate and every point was expressed as mean±SD.
Biomolecules media composition
To evaluate design media composition and compare against their controls, FTIR spectroscopy was used. FTIR spectroscopy is a novel tool widely employed to study organic composition of complex systems and allows a fast results obtaining without sample destruction.5,6 It was made a semiquantitative determination of the main biomolecules (proteins, lipids, carbohydrates). These biomolecules are used by strain as nutrient sources. For spectra acquisition, a PerkinElmer GX 1 spectrophotometer was used. 5μL of Media (designed and controls) were processed as liquid in AgCl circular window, dried under nitrogen flow previously. Spectra were collected with 64 scans and 4cm‒1 of resolution within mid‒infrared range (4000cm‒1–400cm‒1). Each media was processed twice and then averaged. Smoothing and base line correction were applied over spectra as pre‒processing step to improve the signal to noise ratio.7 Obtained spectra were analyzed by OMNIC version 8.3 (Perkin Elmer, Waltham, MA) and Origin Pro 8.5.1 software (Electronic Arts, Redwood City, CA). According to the IR active vibration of the biomolecules bonds spectrum regions analyzed were: Lipids (3000–2800cm‒1), Ester bonds (1770–1720cm‒1), Amide I (1700‒1600cm‒1), Amide II (1600‒1450cm‒1), Carbohydrates and polysaccharides (1200‒900cm‒1).8 Amide I and II are directly related with protein composition and absorbance from each spectral region is correlated to biomolecule composition, while lipids and ester bonds regions indicate bacterial bio membrane presence.7,8
Productive activity is a fundamental column in country’s economic development, also is one of the main factors of unsustainability. One of the greatest advantages of pharmaceutical biotechnology is the versatility. And although its approach to the production of drugs opens a plethora of green and sustainable possibilities, the generation of waste continues to represent a problem on a smaller scale, but it can be brought to near zero. Waste production and natural resources consume diminishing sustainability and economic growth. We are seeking to improve our own process and to insert Clean Production in our production lines. When protein concentration in RBC was determinated (Figure 1), non‒significant differences were found between lysates. Also, they present values 10% higher than other lysates presented as protein supplementary.9,10 By using protein concentration as indicator, 12 media were designed and tested for growing compared to commercial media. In all cases, protein sources were replaced for RBC. Table 1 shows all media designed by using RBC from lactic acid bacteria and P. fluorescens. 12 media were designed and tested for bacterial growth. Figure 2 shows Lactobacillus alimentarius growth in four media designed (BCM1 to BCM4) compared to commercial media. Significant differences were found in L. alimentarius growth when BCM1, BCM2, BCM3 and BCM4 were compared to MRS. In all cases, L. alimentarius growth was consistently superior, being 20.53% higher for BCM1, 30.22% higher for BCM2, 27.48% higher for BCM3, and 30.28% higher for BCM4. In the analysis by FTIR spectroscopy of the media composition we could conclude that RBC increases the lipid and carbohydrate sources as it can see in the Lipids and Carbohydrate spectral regions for BCM 1 to BCM 4. While the increases of protein quantity were only observed in BCM1 to 3 Figure 5. Figure 3 shows Enterococcus faecium growth in five media designed (CaGB1 to CaGB5) compared to commercial media MRS. Significant differences were found in E. faecium growth. In all cases, we observed much better growing when we use our designed media: 14.47% higher for CaBG1, 13.76% for CaBG2, 12.00% for CaBG3, 15.68% for CaBG4, 3.34% for CaBG5. As occurred with BCM design media, RBC increases the lipids and carbohydrate sources for CaGB1 to CaGB5. Only CaGB1 and CaGB2 increase the quantity of protein Figure 5. Berg et al. suggest that the presence of peptides in yeast extract enhances growth of Lactobacillus.11 The nutritional requirements have been discussed by Rogosa et al. They emphasized the importance of amino acids and vitamins for the growth of Lactobacillus. 12Amrane and Prigent and Nancib et al. explained the contributions of B vitamins, purine and pyrimidine bases in the medium for the growth of Lactobacillus.13,14 Our results show that, these necessities could be completely supplemented by using RBC. Figure 4 shows Pseudomonas fluorecens growth in three media designed (GS1 to GS3) compared to commercial media Luria Bertani broth. Significant differences were found in P. fluorescens growth. In all cases, we observed much better growing when we use our designed media: 32% higher for GS1, 9.67% for GS2, 35% for GS3. This increase in the concentration of biomass allows us to infer an increase in the cellulose production by Pseudomonas fluorescens. It is reported that increases in bacterial growth generate more exopolysaccharides.15 RCB contributes increasing lipids and carbohydrates for GS1 to GS3 Figure 5. Frequency displacement observed in AMIDE regions for GS1 to GS3 respect from LB control, indicates a change of protein sources in the media composition, and could be responsible for the higher growing rates obtained in the design media Figure 5.
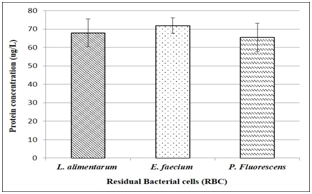
Figure 1Protein concentration for RBC from L alimentarius, E faecium and P fluorecens, measured as ug/L by using bovine albumin as positive control.
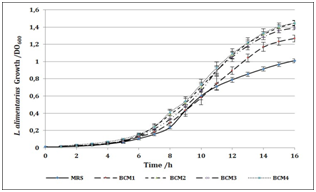
Figure 2L alimentarum growth measured as OD600 vs time in media samples BCM1, BCM2, BCM3 and BCM4 and MRS commercial media as positive control.
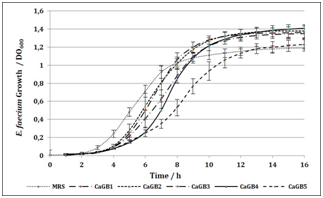
Figure 3E faecium growth measured as OD600 vs time in media samples: CaGB1, CaGB2, CaGB3, CagB4, CaGB5 and MRS commercial media as positive control.
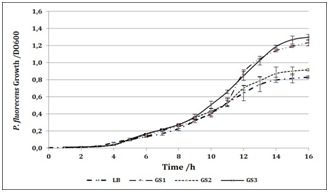
Figure 4P fluorecens growth measured as OD600 vs time in media samples: GS1, GS2, GS3 and LB commercial media as positive control.
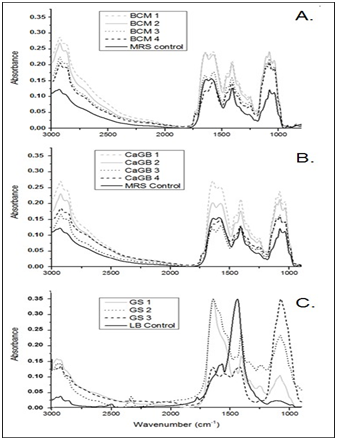
Figure 5FTIR Spectra of design media and controls. A. Shows BCM 1 to BCM 4Vs. MRS Control. B. Shows CaGB 1 to CaGB 4 Vs. MRS Control and C. Shows GS 1 to GS 3 Vs. LB control.
Strains bacterial |
Media designed* |
% protein replaced |
BRC (g/L) |
YE |
BE |
BP |
Salt |
Glucose |
pH |
L. alimentarious |
BCM1 |
50 |
2.5 |
‒ |
X |
X |
X |
X |
6.51 |
BCM2 |
100 |
5 |
‒ |
X |
X |
X |
X |
6.47 |
|
BCM3 |
50 |
7.5 |
‒ |
‒ |
X |
X |
X |
6.31 |
|
BCM4 |
50 |
10 |
X |
‒ |
‒ |
X |
X |
6.23 |
|
E. faecium |
CaGB1 |
50 |
2.5 |
‒ |
X |
X |
X |
X |
6.46 |
CaGB2 |
100 |
5 |
‒ |
X |
X |
X |
X |
6.37 |
|
CaGB3 |
50 |
7.5 |
‒ |
‒ |
X |
X |
X |
6.32 |
|
CaGB4 |
50 |
10 |
X |
‒ |
‒ |
X |
X |
6.18 |
|
CaGB5 |
50 |
7.5 |
‒ |
X |
‒ |
X |
X |
6.35 |
|
P. fluorecens |
GS1 |
50 |
2.5 |
‒ |
‒ |
X |
X |
‒ |
6.52 |
GS2 |
50 |
2.5 |
X |
‒ |
‒ |
X |
‒ |
6.45 |
|
GS3 |
100 |
7.5 |
‒ |
‒ |
‒ |
X |
‒ |
6 |
Table 1 Media growth culture's composition
We achieve our main purpose of design a non‒waste process production line, by transforming remnant biomass in higher values products. We develop 12 growth media, and its innovation degree allow as also, there intellectual protection on a patent INPI ARN20170102314. In addition, we will reintroduce the products obtained in the production lines as substitutes for the commercial protein and carbohydrate sources, in order to create a green interface and to reduce costs. We achieve our main purpose to minimize environmental damage, to use resources more efficiently; and to increase business profitability and competitiveness.
National Agency for Scientific and Technological Promotion (ANPCyT), Ministry of Science and Technology (MiNCyT), National Council for Scientific and Technical Research (CONICET).
Authors declare there is no conflict of interest.

©2018 Eugenia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.