MOJ
eISSN: 2575-9094


Review Article Volume 2 Issue 5
1Centre For Human Reproduction, National medical university , India
2A centre for human reproduction672,kalpak garden, perry cross road, near otter’s club, bandra(w)-400040, India
3Department of Neurologist, Swami Satyanand Hospital Near Nawi Kachehri, Baradri, Ladowali road, India
Correspondence: Kulvinder Kochar Kaur, centre for human reproduction721, g.t.b. nagarjalandhar-144001punjab, india, Tel 91 181 9501358180
Received: August 01, 2018 | Published: September 12, 2018
Citation: Kaur KK, Allahbadia G, Singh M, et al. Advances in BAT physiology for understanding and translating into Pharmacotherapies for obesity and comorbidities. MOJ Drug Des Develop Ther. 2018;2(5):166-176. DOI: 10.15406/mojddt.2018.02.00057
With obesity growing in epidemic proportions and very few medical drugs that can be used for longterm there has been a need for some pharmacotherapy that can help maintain weight loss on longterm basis. Thus we made a PUBMED search for articles related to BAT metabolism in obesity using the MeSH terms, brown fat, beige, brite adipocytes, activation of BAT, cold induced activation, mechanism of action and found 1400 articles of which we selected 164 articles for this review. Duplicate articles were not included and those reviewed in our earlier articles were also not included. No meta analysis was also done. We identified different kinds of drugs capable of activating brown asipocyte (BAT) thermogenenesis and classified them into 4 groups, with group 1 acting on beta3 adrenoceptors, group 2 on noradrenaline uptake, 3 on peroxisome proliferator‒activated receptor (PPAR)‒gamma which included mostly the thazolidinedione group currently used for diabetes treatment of which pioglitazone is the most common one although not much studies have been done to study their action on BAT thermogenesis. Confirmation of BAT activation was done with use of 18‒F‒fluorodeooxo glucose by PET/CT Studies. Of class 1 mirabegron offers most promise be in used currently for overactive bladder. Many miscellaneous drugs like caffeine, nicotine, curcumin, capsaicin, forskolin, FGF21 have been considered as class4. Further role of BAT transplantation has been highlighted with very little BAT available for drug action to be of help in treating obesity.
Keywords: thrmogenesis, brown adipocytes, 18F‒FDG, mirabegron, pioglitazone, PPAR‒gamma, curcumin, caffeine, nicotine, CB1 receptors
According to the WHO more than I billion adults are overweight and of these at least 200million men and 300million women are clinically obese.1 In a prospective study where over 9million people were evaluated in the last 3 decades ,it was found that the average body mass index (BMI) increased by 0.4‒0.5kg/m2/decade and subregion trends showed that the average BMI increased by 1.4kg/m2 in men and 1.9kg/m2 in women/decade.2 Obesity has been found to be a major risk factor for many diseases like cardiovascular diseases (CVD), type 2 diabetes mellitus (T2DM), stroke, hypertension and many cancers.3 Special emphasis has been laid on finding strategies by which energy expenditure can be increased ,with the discovery of brown and beige adipocytes in humans. Earlier we had summarized the location, differentiation, surface markers of brown adipose tissue (BAT) /Beige adipocytes and mechanisms other than cold/β3 adrenergic agonists by which they can be targeted to improve obesity and thus metabolic syndrome (MS) and other comorbidities.4‒6 Here we further try to update how one can use enhancement of Brown adipose tissue( BAT) /Beige Brite adipocytes in the management of obesity and T2DM.
Methods Thus we made a PUBMED search for articles related to BAT metabolism in obesity using the medical subject heading (MeSH) search terms, brown fat, beige, brite adipocytes, activation of BAT, cold induced activation, mechanism of action, treatment strategies with relevance to BAT.
Results We found 1400articles of which we selected 164articles that were peer reviewed for this review from 1986 to 2018 .Duplicate articles were not included and those reviewed in our earlier articles were excluded. We examined all abstracts for relevant information about the pertinent topics. Additional literature was retrieved from references and cross references. No meta analysis was done.
Brown fat cells also have enzymes to synthesize and store triglycerides, although in BAT fat cells lipids are stored in multiple small fat droplets i.e they are multiloculated in contrast to white fat cells which are unilocular, containing one giant droplet of triglycerides. Besides BAT is composed of brown fat cells, along with plenty of blood vessels and nerves. In brown–type fat oxidative phosphorylation is uncoupled, so that the process of respiration is inefficient allowing for significant heat production (non shivering thermogenesis) through consumption of calories without ATP production, which is facilitated by up regulation of thermogenic promoting factors which includes uncoupling protein 1(UCP1).7 Typical brown adipocytes are present between the scapulae of rodents ,and in neck region of humans.8‒10 Figure 1 Recently interest has emerged towards development of pharmacotherapies which try to augment brown adipose tissue (BAT) to raise energy expenditure following the accidental discovery and presence of BAT in adult humans as well besides their presence in smaller mammals and human infants, when on using 2‒deoxo‒2‒Fluoro‒D–Glucose (18F FDG)/ positron emission tomography (PET) scanning in cancer patients several discrete areas of metabolically active BAT was suggested.11 Further recent data indicate that normal adult humans contain significant depots of UCP‒1 positive brown fat which can be detected by 2‒deoxo‒2‒ Fluoro‒D–Glucose (18F‒FDG)/ positron emission tomography (PET) / computed tomography (CT) scanning methods specially in supraclavicular and neck area (biopsy proven) (Figure 1).12‒16
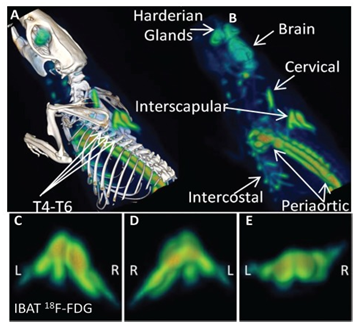
Figure 1 Courtesy ref no 164‒PET Images from Class1 drug Effects on Rat BAT‒CL316,243 induced activation of BAT is seen in the PET image (A) regional localization confirmed by PET/CT (B) Interscapular, periaortic, cervical and intercostal BAT regions are evident. The bilateral structure of activated interscapular BAT (IBAT) is evident in the ventral (C), dorsal (D), caudal (E) views of IBAT.
Positive transcriptional regulators
Were Forkhead box C2 (FOXC2) and PR (PRD1‒BF‒RIZ1 homologous) domain containing 16 (PRDM16) although only PRDM 16 was supposed to determine brown cell fate in a cell autonomous manner.17,18
PR (PRD1‒BF‒RIZ1 homologous) domain containing 16(PRDM16)
Drives a fat differentiation programme. To understand the mechanism by which PRDM16 activates brown fat selective genes, chromatin immunoprecipitation (ChIP)followed by deep sequencing (ChIP seq)analysis in BAT revealed that PRDM16 binding is highly enriched at a broad set of brown fat selective genes. PRDM16 physically binds to MED1 a component of the Mediator complex and recruits it to superenhancers at brown fat selective genes. Deficiency in PRDM in BAT reduces MED1 binding at PDRM16 target sites and causes a fundamental change in chromatin architecture at key brown fat selective genes. Thus PRDM controls chromatin architecture and superenhancers activity in BAT. PRDM16interacts with MED1 at brown fat specific genes to promote gene transcription .The binding of MED1 to PRDM16 appears to be direct since these factors are able to bind together in vitro as purified proteins. MED 1 is a component of the Mediator complex which plays akey role in regulatory genes expression through a variety of mechanisms.19 Mediator bridges enhancer regions and associated transcription factor complexes with RNAII polymerase and the transcriptional machinery at the promoters.19 Harms et al suggest that PRDM16/PRDM3 binds to chromatin at enhancers, many of which are super enhancers(SE) in BAT selective genes via peroxisome proliferator activated receptor γ (PPARγ) and CCAAT/enhancer binding protein (C/EBP β) and likely other factors. At these sites PRDM16 recruits MED1/Mediator and by doing organizes higher order chromatin architecture and promotes pre initiation complex assembly to target gene transcription. A loss of PRDM6 and MED1 dirsrupts higher order chromatin architecture at certain brown specific target genes without impeding the chromatin binding of other transcription factors, including PRDM16 interacting partners like PPAR γ and C/EBP β. The result of Harms et al indicated that AT ppargac α PRDM16 facilitates an active chromatin hub that links at least two enhancer elements of the promoter region, differentiation of at certain brown fat specific target genes without impeding the chromatin binding of other transcription factors, including prdm16 interacting partners PPARγ and C/EBP and likely other factors. At these sites PRDM16and MED1disrupts a higher order chromatin architecture.20 Furthermore Lida et al demonstrated a direct interaction of PRDM16 with MED1 subunit of the Mediator complex through the zinc finger domains. This gets recruited by enhancer of brown fat specific UCP1 genes through this interaction the enhancer of the thyroid hormone receptor (TR) driven transcription in a biochemically defined system in a mediator dependent manner.21 Further reviewed in Seale 2015 (Figure 2).22
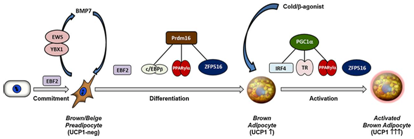
Figure 2 Courtesy ref no54‒Transcriptional modulation of brown fat cell differentiation and activation,Ebf2 marks the committed brown adipocytes and regulates the commitment process from upstream stem cells.EBS/YBX1 regulates BMP7 production which then acts in an augmented manner inducing brown adipogenesis.Ebf2,PRMT, ZFP516 specifically regulate the induction of brown specific genes. The differentiation process of PRDM 16 co activators, C/EBP β, PPAR γ, PPAR α, thyroid receptor (TR) and ZFP 16.Upon cold exposure, β adrenergic agonist treatment of brown fat cells are activated to undergo thermogenic and beige adipocyte expression of thermogenic genes.IRF4 plays a major role in process through recruiting PGC‒1α coactivator,PGC‒1α can also coactivate PPAR’s and TR to activate the transcription of thermogenic genes.
Recently De Sousa et al 2015 have shown that p107 is a transcription factor crucially required for the determination of adipocyte lineage fate choice of stem cells. p107 is strictly expressed only in white adipocyte stem cell compartment, while p107 deficient stem cells give rise to brown adipocytes always both in vitro as well as in vivo. Brown fat programming of mesenchymal stem cells by PRDM16 was associated with a marked reduction in p107 levels. PRDM16 directly suppressed p107 transcription via promoter binding.23
Role of ewing sarcoma protein (EW S)/ Y box protein 1(YBX) / bone morphogenetic protein7 (BMP7)
Recently Park et al showed the role of multi domain protein Ewing sarcoma protein (EWS) which is a an RNA binding protein as a regulator of adipogenesis, upstream of BMP7.It acted in conjunction with Y box protein 1(YBX1)to induce the transcritptional activation and production of BMP7 (Figure 2).24 Earlier Tseng et al had already shown that BMP7 is an important upstream regulator of PRDM 16 in brown preadipocytes.25 Importantly Ewings null mutant BAT and beige preadipocytes ectopically expressed myogenic genes and the treatment of these with BMP7 could lead to a full rescue of brown adipogenic differentiation capacity. Further Park et al recommended to determine how this EWS/YBX1 expression activity is regulated to further elucidate brown fat lineage determination to plan treatment at an upstream level.24 Different studies have reported on increased metabolic activity of BAT Uptake of 2‒deoxo‒2‒18F‒Fluoro‒D–Glucose(18F‒FDG), In human BAT positron emission tomography /computed tomography (PET/CT) studies.26‒28 Simultaneously ways were found of examining BAT in rats with the use of 123+I MIBG, that is an analog of norepinephrine,29 followed by norepinephrine trasporters, which got visualized with the use of 11C –Methyl reboxitine (MRB) and 11C‒ [4‒methylamino‒4′‒N, N‒dimethylaminoazobenzene (TAZA).30,31 Use of 11C –acetate,11C‒palmitate along with using radiolabelled fatty acids as metabolic substrates was done by other researchers.32,33 For getting newer strategies to be able to act regarding obesity therapy it is important to be able to measure the metabolic activity of BAT. Activity of BAT is indicated by the deposition of various metabolic substrates like 18F‒FDG, 11C–acetate,11C‒palmitate that is secondary to UCP1 activity,34 and stimulation of thermogenic function.3 This activated BAT might be used to develop strategies for fighting diabetes ,obesity besides hyperlipidemia.35 Imaging studies using 18F‒FDG PET/CT supports the biology of BAT in both humans along with animals. Increased 18F‒FDG uptake occurs in cold activated BAT both in humans (at160C) as well as in rodents (‒40C).36,37 Previously for exploring BAT exposure to cold for long duration was the only way of studying BAT prior to PET ,which was a function mediated by β‒3 adrenergic system.36 The BAT amount varied from 30%‒95%,that is much more than that found in retrospective studies.36,38,39
Activating BAT at ambient temperature needs various pharmacological agents,40‒43 which had been reviewed by various workers.44‒46 Drugs used have been divided into 4 groups depending on their main site of action (Figure 3). Class 1 drugs represent the β‒3 adrenergic receptor (AR) agonists that act on the β‒3 AR, which are located on the adipocyte cell surface. Both in animal and human studies they have been used.2) Class2 drugs are drugs which act by altering norepinephrine levels, either by directly mimicking norepinephrine effects or by blocking the effect of norepinephrine transporter (NET) which are located on the sympathetic nerve terminal.3) Class3drugs activate peroxisome proliferator activated receptor‒γ (PPAR‒γ) and activate within the adipocyte 4) Class4 are drugs like natural products with limited information, that is growing now.
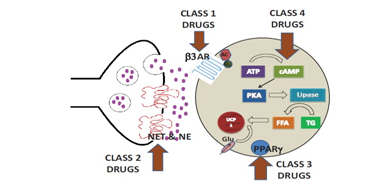
Figure 3 Courtesy ref no 164.‒ Schematic of Sites of Drug Action: Class 1 drugs act on the adipocyte cell membrane bound _3 adrenergic receptor (_3AR) triggering a cascade of events via c AMP. Class 2 drugs act on the norepinephrine transporter (NET) on the sympatheic nerve terminal and increase norepinephrine (NE) levels which then stimulates _3AR. Class 3 drugs activate Peroxisome proliferator‒activated receptor gamma (PPAR). Class 4 drugs act on various pathways within the a dipocyte.
Class 1 drugs
Agonists for β‒3AR at present are being used for overactive bladder(OAB).47 These β‒3AR belong to the G–protein coupled receptors (GPCR) and are present in big quantities on brown adipocytes.48‒51 Sympathetic nerves which contain norepinephrine ,innervate BAT, activate β‒3AR.Alot of work has been used to examine these β‒3AR by developing selective agonists for treatment of obesity.52 Cohen et al have recently reviewed how both brown and beige fat may be molecular parts of the same thermogenic machine. In both UCP1 dependent thermogenesis are required for the generation of heat in cells expressing lipids and carbohydrates by the leak of protons back across the inner membrane of the mitochondrial membrane by UCP1 (Figure 4).53 They further highlighted the importance of factors like early B cell factor 2 ( Ebf2) up steam of Prdm16 promoting binding of PPARγ to the promoters of BAT selective genes and role of euchromatic histone methyl transferase (EHMT1), ahistone methyl lysine transferase in the PRDM16 transcription factors complex which controls the adipose cell fate and loss of EHMT1 ,causes severe loss of brown fate characteristics ,while inducing muscle differentiation in vivo.54 Similarly beige adipocytes also have selective factors upstream of PRDM 16,besides beige specific epigenetic regulators and modulators.TLE3 being a cofactor which belongs to the groucho family as a transcriptional integrator of the PPAR γ and wingless and INT 1 proteins (Wnt) pathways. It competes with PRDM16 for binding to PPARγ and thus can modulate both white versus brown/beige phenotype. Over expression of transduc in like enhancer of split3 (TLE3) can impair thermogenesis while deletion enhances thermogenesis in brown and beige fat.55,56
β‒3AR selective agonists are derivatives of ‘’2hydroxy ethylamino’’ backbone which stimulates norepinephrine.BRL37344,which is an active metabolite of BRL35135 has selective activity for adipocyte lipolytic response.57 2‒deoxy‒[3H]‒glucose is used to study glucose utilization index (GUI) of BRL35135.Treatment with BRL37344 chronically leads to a 34 fold rise in basal GUI of BAT ,without any effect on GUI on other tissues.58 BRL35135 also improved glucose tolerance in genetically obese (ob/ob)and obese zucker (fa/fa)rats at doses ineffective with anti obesity activity.59 Another β‒3AR selective agonists is CL316,243which is pharmacologically (RR)‒5‒[2‒[2,3‒(3chlor phenyl)‒2‒hydroxy‒ethyl‒amino]propyl]‒1,3‒benzodioxole‒2,2cicarboxylate,disdium salt.42,60 It causes activation of cervical, periaortic, intercostals and interscapular BAT(IBAT)as has been demonstrated by PET studies (Figure 2).16 Since this drug acts selectively, one can draw conclusions that increase in 18F‒FDG uptake is secondary to stimulation of β‒3AR,as has been seen with the actions of CL316,243 on overall energy expenditure in BAT.41 Its effects are raised in BAT mitochondrial multiplication along with energy expenditure, that is mainly affected by fast changes occurring in uncoupling protein 1(UCP1)intrinsic action that is secondary to sympathetic stimulation.61 When tried on humans the effects on energy expenditure following 8weeks of use of CL316,243 in young lean male subjects was no different from baseline.62 Thus use in humans was given up in view of its poor bioavailability. There are drugs which are similar structurally like mirabegron, rafabregon and solabegron that are being tried for use in overactive bladder (OAB) and irritable bowel syndrome(IBS).47 Rafabregon causes rise in energy expenditure, of the magnitude of 50kcal/day at highest dose in obese people,63 in both sexes. Effects of solabegron has not been studied for energy expenditure (EE) although it is being used for irritable bowel syndrome ( IBS). Mirabegron, is being used for OAB,64 and is a β‒3AR selective agonist.65 It activated inguinal BAT (IBAT) metabolic activity ,both in rat,66 along with in human,67 that was measured with the use of 18F‒FDG PET/CT. Hence this rise in glucose metabolism in different species might be utilized for treatment of obesity along with T2DM. Talibegron or ZD 2079 and ZD 7114 belong to groups of drugs that have selective β‒3AR activity and cause rise in EE by nonshivering along with decreased gain in weight, along with thermogenesis that is activated.68 Besides that ZD 7114 also has been found to have antagonistic activity in these β‒3AR’s in isolated rat ileum,69 causing no change in 24h EE in obese people although talibegron demonstrated some little stimulatory action on EE, thus it doesn’t offer much promise for Obesity/T2DM. Other β‒3AR agonists which get used in clinical scenario are amibegron or SR58611A,70,71 that were used as antidepressants but were stopped. One finds expression of β‒3AR in BAT, white adipose tissue (WAT), myocardium ,skeletal muscle and liver.50,72 But, in brain expression of β‒3 adrenoceptors was lower as compared to that in BAT.73 One can’t say if poor performance of amibegron is related to small concentrations of β‒3AR or not.
Class 2–drugs changing norepinephrine
It is known that Norepinephrine stimulates β‒3AR and that cold temperature might be increasing metabolism by raising Norepinephrine levels.74,75 Increasing the dosage of Norepinephrine caused an increase of 2‒[3H]‒DG(Glucose metabolic index) in BAT .This implies that norepinephrine raises the capability for BAT thermogenesis.76 Mice where UCP1 is ablated, adding norepinephrine to brown adipocytes lead to an increased oxygen consumption rate. Ephedrine that has structural similarity to norepinephrine, also increases BAT activity but only in lean and not obese participants. Comparing to placebo the BAT changes caused by ephedrine had a negative correlation with different body fat indices.77 Although chronic ephedrine therapy decreased body fat content, there was no association with a rise in BAT activity. But since there was decrease in BAT glucose disposal with the use of chronic ephedrine, it gave suggestion that this therapy reduced instead of increasing BAT activity.78 For the treatment of attention deficit hyperactivity disorder (ADHD),there is use of atomoxetine, that is a very efficacious and selective presynaptic NET blocker.79 Atomoxetine in lieu of rise in adrenergic neurotransmission causes raised synaptic concentrations of norepinephrine.80 Since a very selective NET ligand ,11C‒MRB is taken up by BAT, there is suggestion that there are transporters present in BAT.30 Also 11C‒TAZA via the NET in the BAT along with IBAT and other BAT regions also gets taken up as is shown by PET.31 Quantification of atomoxetine’s effects on BAT metabolism was done using 18F‒FDG PET/CT recently.81 Much more increase occurs as compared to ephedrine.40 Since propranolol inhibited atomoxetine induced BAT activation to control levels ,it suggests that action of atomoxetine is through β‒3AR.Initial reports showed short term antiobesity efficacy ,causing modest short term weight loss in obese women.82 In case of binge eating disorders, atomoxetine was found to be useful in outdoor patients.83 But no weight lowering effect was seen in patients having gained weight with the use of clozapine or olanzapine.84 Another potent and selective inhibitor of NET uptake, i.e. nisoxetine bound Inguinal brown adipose tissue (IBAT).85 Similarly binding of IBAT density from angiotens an II infusion lead to good weight lowering effects ,which was secondary to raised sympathetic transmission.86 Although sibutramine ,which is also another NE T reuptake inhibitor showed thermogenic effects ,it was removed from the market because of its CVS side effects.87 Treatment of fibromyalgia patients with another NET reuptake inhibitor, milnacepram showed roughly 5% weight loss in 3‒6months.88
Class3‒PPARγ activators
Once PPAR γ is activated by thiazolidenediones, they affect both lipid along with carbohydrate metabolism by various mechanisms when used for treatment of T2DM.89 Since brown adipocytes increase energy expenditure, increase of brown fat adipogenesis could explain the good effects of these drugs in insulin sensitivity in humans. Rosiglitazone helps in proadipocyte cell line differentiation and helps in increasing IBAT mass. Once human preadipocytes were prepared from all depots that got rosiglitazone, there was an increase in UCP1 mRNA.90 Also troglitazone another thiazolidenediones was shown to increase IBAT in rodents who got treatment with troglitazone.91 But ciglitazone, although it caused reduction in blood glucose ,triglycerides and food intake it did not change body weight in obese hyperglycemic mice. Though it did cause a reduction in blood sugar, currently it is not being used for T2DM treatment in any form of medication.92 GLUT4 expression gets increased by troglitazone in T2DM obese rat models and increases insulin sensitivity in non insulin dependent DM although with severe liver side effects.93 Currently pioglitazone is getting used to treat T2DM although it has some urinary bladder side effects in some cases.94,95 In a rat model it plays a role in remodeling of adipocytes.96 Balaglitazone decreased glucose levels without having any effect on fluid retention/bone formation in rats that were obese. It improved blood glucose levels along with HbA1c in diabetic patients.94,95 Another drug from this group rivaglitazone decreased glucose levels by causing better insulin sensitivity in T2DM animals taking a small time. Clnical trials with rivaglita zone are under way for T2DM therapy and assess what risks could be associated with this drug.97,98 Increase in BAT was caused by darglitazone, causing changes in morphology in rats.99 But development of darglitazone for use in clinical arena has been stopped. Hence of all the glitazones, pioglitazone is the most active, though it decreases blood sugars, whether it causes browning of adipocytes and helps in weight loss reduction has yet to be proved. There have not been any PET imaging studies for studying BAT activation in either animal or human models has been done. There is need to study whether in vivo BAT gets activated by using pioglitazone and that needs to be compared with class1drug miraberon.
Class 4‒natural [roducts /other drugs
Catecholamine release follows intra‒peritoneal nicotine injection like norepinephrine that stimulates thermogenesis in BAT for energy expenditure.100 18F‒FDG uptake in BAT was increased by nicotine ,an effect that got more increased by Addition of ephedrine.41 Thus nicotine increases norepinephrine turnover along with BAT thermogenesis, and simultaneously increasing resting metabolic rate, all add to reduced obesity.101 Caffeine increased oxygen consumption in BAT mitochondria, and although increased BAT temperature it did not have that much effect on core temperature.102 Also adenosine receptors A2A also have been shown to take a part in BAT thermogenesis.103 Still it is not shown if there is interaction of adenosine receptors with caffeine is involved in BAT activity. Capsinoidsor capsaicin has been known to reduce body fat. Energy expenditure (EE) increase caused by capsinoid is due to BAT involvement, as seen by studies in small rodents. Also 2 weeks of capsinoid treatment caused increased UCP1 expression.104 In humans also increase in energy expenditure ( EE) occurs by activation of brown adipose tissue. Turmeric pigment curcumin has been studied for treatment of obesity related diseases. Its action is by acting directly with adipocytes, pancreatic cells, hepatic stellate cells, macrophages and muscle cells. It reverses insulin sensitivity, hyperglycemia, hyperlipidemia along with other obesity symptoms. Besides that it can bind to PPARγ by which it can stimulate human adipocyte differentiation.105 It also improves cold tolerance in mice along with expression of β3 adrenoceptor gene in inguinal WAT. Increase in norepinephrine levels was also observed with curcumin therapy.106 Forskolin induces thermogenic response in BAT.107 Adenylylate cyclase enzyme gets activated directly by forskolin which further leads to increase in cyclic andenosine monophosphate (cAMP).93 This increased metabolism of BAT by forskolin in measurable by 18F‒FDG PET/CT.15 Another drug rimonabant ,a cannabinoid 1( CB1) receptor drug lead to weight loss by causing increased BAT temperature by which it mediated weight loss through the peripheral endocannabinoid system ,as proved by the use of peripheral CB1 receptor antagonist AM6545.108,109 Rimonabant got banned because of the suicidal side effects observed by its use.110 Still by using peripherally acting CB1 receptor drugs like AM6545 in PET imaging might help in further highlighting the role of this target receptor. A selective Kv1.3 peptide inhibitor ShK‒186, shows marked effect in the treatment in a mouse model of diet induced obesity and insulinsensitivity.111 BAT activation is caused by ShK‒186 is proven by increased glucose uptake, increased β‒oxidation and raised transcription of UCP1 gene that is involved in BAT thermogenesis. ShK‒186 decreased increase in weight even after voracious diet consumed in mice fed an obesity inducing diet. Increased membrane re modelling along with simultaneous increase in PPARγ expression and metabolites which can cause activation of PPARγ might explain how this drug helps. As there is improvement in insulin sensitivity along with control of T2DM with PPARγ agonists,112 increased PPARγ signaling seen in mice receiving ShK‒186 for treatment might be responsible for this peptides beneficial effects in treatment.
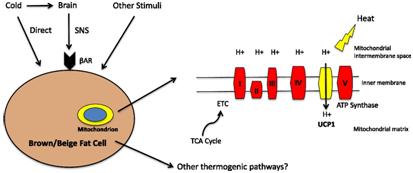
Figure 4Courtesy ref no‒89 Schematic adaptive thermogenesis in brown and beige adipocytes .This process is typically thought of as beige indirectly activated by cold via the sympathetic nervous system (SNS)catecholamine stimulated adrenergic receptors(BAR) ultimately activating UCP‒1 dependent thermogenesis. Adaptive thermogenesis can also be directly activated by cold in beige adipocytes and by other stimuli that may signal independently from the β adrenergic receptors. The reducing equivalent generated by the tricarboxylic acid (TCA)cycle enter the ETC(electron transport chain ).This generates a proton gradient across the inner mitochondrial membrane i9nstead of linking this gradient to ATP synthesis via complex V .UCP1 is able to uncouple this gradient with the chemical energy to heat.
Class 1 drugs
Since in human BAT there is presence of β‒3AR, it suggests therapeutic strategies can be used on this basis.72 Still there are problems with regard to selectivity and bioavailability of the drugs along with measurable weight loss with class1drugs are not fully clear. CL316,243 possesses only 10 fold selectivity for human β‒3 over β‒2 adrenoceptor and also β‒3AR mRNA is also seen in human heart,113 because of which one gets worried regarding the CVS side effects. Though with CL316,243,there are no changes in heart rate( HR),systolic and or diastolic blood pressure, no changes in ECG intervals have been seen nor any tremor development was observed.48 Although the newer drugs like mirabegron which also target this receptor have the food and drug administration(FDA) approval for OAB, still it is not clear what is their role in treating T2DM.39 Use of CL316,243 chronically has been shown to effect obesity in mice and rats.33,114,115 Also acute β‒3AR stimulation with the use of CL316,243 increases BAT metabolism in vivo which is measurable quantatively with 18F‒FDG PET/CT by observing 18F‒FDG uptake in rats .Once exposed to cold temperature, In early phase, there is mobilization of fatty acids from WAT, that is known to be the initial source for activation of BAT instead of breakdown of fat depots stored in BAT.116,117 On histology the number of lipid vacuoles in BAT was significantly reduced following stimulation with CL316,243,though no change in WAT lipid content was seen between the 2 situations.29 Thus during acute administration of CL316,243,glucose metabolism along with stored lipids lipolysis in BAT are the initial ways by which there is activation of tissue instead of mobilization of fatty acids from WAT .Despite similar binding of β3AR in humans as well as rodent receptor in vitro, in human β3AR it just remains as partial i.e 60% agonist, in contrast to it being a full agonist, having a poor bioavailability, only 10% of it getting absorbed orally.48 In Zucker lean (ZL) and Zucker obese(ZF) rats the difference in β3 adrenoceptor agonist mediated activation of BAT was investigated with the use of 18F‒FDG PET/CT. Brain 18F‒FDG PET studies had been used in ZF model to study the leptin receptor deficiency centrally.118,119 There was a 4 fold BAT activation in ZL in comparison to 2 fold in ZF rats as compared to saline.120 This reduced activation goes parallel with the lower β3 adrenoceptor levels in ZF rats. Although there were lower β3 adrenoceptor levels along with decreased G‒protein coupling in ZF rat model ,measurable effect of CL316,243 was seen on BAT. Considerably lower opacity was seen in ZF in contrast to ZL as seen by CT ,which suggests that there is low abundance of brown adipocytes in the IBAT region. Further attempt is being done for developing treatment strategies where leptin receptor function is restored regarding human obesity therapeutics.121 Conservation of residual β3AR is seen in the leptin deficient fa/fa rat model, which is functional with respect to enhancing metabolic activity. Along with that there is decreased coupling of β3AR with the G protein in white adipocytes.122 Other pointers which impair BAT thermogenesis is abnormalities in central metabolism regulation and neuroendocrine metabolism.123 Studying chronic β3AR drug treatment in this rat model might help in restoration of brown adipocytes. Streptozoc in treated rat model is used to study type1 diabetes mellitus (T1DM),and lower metabolic capacity of IBAT is seen in this model.124 Baranwal et al further verified the loss of metabolic capacity of IBAT in streptozocin diabetic rats.124 Thus difference in the 2 diabetic models is that the decrease in IBAT activity in the zucker fat rat might occur secondary to impaired β3AR signaling while the decrease that is seen in streptozocin diabetic rats impairment might be driven by mitochondrial dysfunction. They further suggested that stimulation of β3AR activates IBAT in this T1DM rat model and can increase metabolic activity. But trying to change norepinephrine levels with the use of atomoxetine did not have much effect, possibly because of impaired norepinephrine turnover .If insulin receptors are blocked in BAT streptozocin –treated mice, it caused impaired glucose tolerance just as that seen in nondiabetic animals which suggested the importance of insulin receptor activity in helping reverse DM.125 Mirabegron, which is a selective β adrenergic agonist ,it is used to treat overactive bladder in a dose of 200mg.This drug has received clinical approval and has been shown to activate human brown adipocytes that might revoke interest in this pathway.67 In contrast to CL316,243,Merabegron has a much better agonist potency as far as humans are concerned.16,64 Another selective β3AR agonist is amibegron which crosses blood brain barrier( BBB),having antidepressant like properties ,like its ability to increase serotonin synthesis.71 So more studies are required to study disease models using these newer human β3AR drugs.
Class 2 drugs
A selective norepinephrine reuptake inhibitor is atomoxetine, that has low abuse potential.79 Structurally it is similar to the antidepressant fluoxetine, and acts by increasing synaptic norepinephrine levels,126,127 having few side effects[CVS side effects rate (3%)and raised blood pressure.128,129 It is already being used to treat ADHD in psychiatry, both pediatric and adult with very little adverse effects.130,131 When fasting there was excessive 18F‒FDG increase in BAT caused by atomoxetine as compared to control.72 In patients with pheochromocytoma ,where there is increased release of epinephrine along with norepinephrine from the adrenal gland, there is intense 18F‒FDG uptake.132,133 Because of intense adrenergic interaction with β1 and β2 adrenoceptors, there are serious CVS side effects seen in these patients.134 Hence specificity for β3AR is required for developing agents for BAT activation.
Sibutramine is a combined norepinephrine and serotonin reuptake inhibitor that had been earlier used for obesity therapy because it decreased appetite along with initiating weight loss along with diet and exercise. It improved insulin sensitivity along with glucose metabolism. All these effects were caused by intrinsic effects of the drug rather than weight loss, but it was withdrawn from market because of CVS side effects.135 Another norepinephrine and serotonin reuptake inhibitor antidepressant is milnacepran, that has been used in comorbid depression that is common in T2DM patients .Improvement of blood glucose and Hb A1c levels occur in T2DM patients. Because of relief of depression, better self care occurs causing improved metabolic parameters,136 with BAT activation protecting from hyperglycemia.137
Class 3 drugs
Because of serious side effects many thiazolidinediones have been given up for BAT activation.13,80 Currently pioglitazone is the one PPAR gamma activator used for T2DM.138 In a recent study India specific algorithm for management of T2DM using pioglitazone was done.139 Still role of BAT has not been demonstrated in glucose lowering effect of pioglitazone, as UCP1 in human epicardial adipose tissue did not change.140 Thus measuring effect of pioglitazone on either animal/human BAT with the use of 18F‒FDG uptake using this imaging method might help to be able to confirm its metabolic activity.
Class 4 drugs
Nicotine ,though activates BAT,141 its weight lowering effect seen with smoking is secondary to its hypothalamic actions.142 Forskolin activates cyclic AMP via adenylyl cyclase activation in multiple cell types.143 Forskolin although activated BAT its action on heart myocardium ,along with adverse effects like headache, decreased BP, increased HR, with limited weight lowering effects don’t make it a good choice. Caffeine has small effects regarding increasing fat metabolism that gets accentuated with use with ephedrine.144 Capsaicin may have role in obesity through brown and beige adipocytes.145,146 Curcumin promotes browning of WAT.147 A bioavailable form of curcumin increased weight loss in overweight people having metabolic syndrome( MS)].148 Besides rimonabant, which has side effects, other peripheral CB1 selective drugs might be useful.149,150 ShK186,is undergoing trials for therapy in autoimmune diseases.151 It had great effects in a mouse model of diet induced obesity.103 Fibroblast growth factor 21(FGF21) is being targeted for obesity and may partially activate BAT.152,153
BAT transplantation
Transplantation of BAT might have edge over pharmacological drugs as BAT levels are quiet low in obese people. Recent reviews cover the advantages of this in improving body composition and metabolism.154 Stem cells for developing AT implants are being explored to overcome problems related to availability of these implants for providing BAT for human therapy purpose.155,156 Thus one can combine these with pharmacologic approaches.
Thus we have reviewed in detail the BAT physiology, its positive transcriptional regulators like FOXC2, PRDM 16, the upstream regulators of PRDM16 like EWS/YBX1 and BMP7and importance of understanding in formulating treatment strategies. The various drugs that might help have been divided into 4 classes, with class1 drugs being the beta 3 adrenergic receptor agonists located on the adipocyte cellsurface, class 2 drugs acting by changing norepinephrine levels ,acing directly or mimicking norepinephrine effects or by blocking norepinephrine transporter, that are located on the sympathetic nerve terminal,class3 drugs acting on PPAR –gamma and class 4 being miscellaneous. Since presence of BAT in adults is low,157‒161 there is problem in utilizing it for changing metabolism. BAT gets activated only once need for thermogenesis is there or it is activated pharmacologically.24,27 In view of its effects potentially,162,53 pharmacological stimulation attempts have increased.162,163 Engineered tissue transplantation along with pharmacologically induced brown adipocyte biogenesis is available now and may pave the development of therapies for obesity and T2DM. Of class1 drugs mirabegron offers great clinical promise, with further clinical trials on it effects on EE and thus weight lowering effect is needed. Of type2 drugs although sibutramine has been withdrawn from the market, atomoxetine is a drug that can be further studied for its effects on EE and thus obesity. Pioglitazone is the drug that is currently being used clinically for treating diabetes mellitus, although its role on EE,BAT metabolism and thus weight loss needs to be studied. Of the class4 drugs curcumin, nicotine, peripheral CB1 drugs need to be further studied for developing as antiobesity targets. Finally BAT transplantation might prove to be more effective than pharmacological agents alone, or might help in combination with them to improve BAT metabolism and thus EE and obesity.
None.
The Author declares that there is no conflict of interest.

©2018 Kaur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.