MOJ
eISSN: 2575-9094


Research Article Volume 2 Issue 4
1Department of Pharmaceutical Chemistry, Dadasaheb Balpande College of Pharmacy, India
2Department of Chemistry, JB College of Science, India
3Department of Pharmaceutical Chemistry, Kamla Nehru College of Pharmacy, India
Correspondence: Debarshi Kar Mahapatra, Department of Pharmaceutical Chemistry, Dadasaheb Balpande College of Pharmacy, India
Received: June 28, 2018 | Published: July 9, 2018
Citation: Mahapatra D, Dadure KM, Shivhare RS. Exploring the site-specific influence of hydroxyl group in ring–b of murrayanine–chalcone on edema reducing potential. MOJ Drug Des Develop Ther. 2018;2(4):190-193. DOI: 10.15406/mojddt.2018.02.00046
Owing to the fact that chalcones have tremendous potential of exhibiting anti‒inflammatory activity and the development of a chalcone analog of murrayanine may have the perspectives of better anti‒inflammatory activity than the parent moiety itself, in the present research, hydroxylated derivatives of murrayanine‒chalcone were rationally designed and synthesized from murrayanine (1) and hydroxylated acetophenones 2(A‒C) using the previously reported protocol and to explore the anti‒inflammatory potential using carrageenan‒induced paw edema method. In addition to it, the establishment of the possible structure‒activity‒relationship (SAR) was done which will be beneficial for the medicinal chemists in further designing target modulators. The compound 3c having para substituted hydroxyl group exhibited noteworthy edema reducing potential. The compounds 3a and 3b presented a lower anti‒inflammatory activity than that of the best active and the standard drug indomethacin. The SAR expressed that the para substitution 3c expressed highest biological activity followed by meta 3b and ortho 3a substitution. The exploration of acute toxicity revealed the safety profile of the compounds and all the compounds were tested at 100mg/Kg body weight. The edema reducing activity may be due to the interaction of the substituents (of murrayanine‒chalcone) with the inflammatory targets like cyclooxygenase‒1/2 (COX‒1/2) and lipoxygenase (LOX). The current study provided some imperative evidence for the better optimization of the chemical structure to attain marked biological activity.
Keywords: biological activities, mahanine, cyclooxygenase, toxicity, benzothiazepine
Murraya koenigii L. (family: Rutaceae), also known as Indian curry plant is having ethnopharmacological importance.1 The plant parts (root, leaf, and stem bark) are known to have several biological activities like anti‒helminthic, carminative, stomachic, febrifuge, astringent purgative, etc.2 These activities are a function of the phytoconstituents of carbazole scaffolds like euchrestine B, mahanine, O‒methylmurrayamine A, bismurrayafoline, murrayanine, bismahanine, koenimbine, mahaninebine, isomahanine, O‒methylmahanine, bispyrayafoline, and mahaninebicine which in scientific studies over the years have represented potent anti‒ulcerogenic, anti‒bacterial, anti‒oxidant, anti‒fungal, immunomodulation, etc.3 Murrayanine is the most imperative, most active, and highly explored phytoconstituent which have been studied by our research group over the years.4 A chalcone derivative, designated by us as “Murrayanine‒Chalcone” was synthesized,5 and a number of heterocyclic derivatives (oxadiazole,6 thiazole,7 thiadiazole,8 hydantoin,9 benzodiazepine,10 pyrazole,11 benzoxazepine,12 pyrimidine,13 benzothiazepine,14 isoxazole,15 3,4‒methylenedioxy,16 and phthalimide,17 were fabricated in order to amplify the biological activities (anti‒diabetic, anti‒inflammatory, anti‒oxidant, anti‒anxiety, antibacterial, anti‒convulsant, and anti‒fungal) by using the most common strategy ‘hybridization’. Chalcone or 1,3‒diphenyl‒2E‒propene‒1‒one comprises of a benzylideneacetophenone scaffold where the two aromatic nuclei joined by a three carbon α, β unsaturated carbonyl bridge.18 This scaffold is easy to synthesize and exhibit diverse options for substitution and their profound capability to exhibit diverse biological activities such as hypoglycemic, hypolipidemic, antihypertensive, anti‒platelet, anti‒arrhythmic, anti‒obesity, anti‒infective, etc.19‒21 Chalcones have tremendous potential of exhibiting anti‒inflammatory activity and the development of a chalcone analog of murrayanine may have the perspectives of better anti‒inflammatory activity than the parent moiety itself.22 In the present research, hydroxylated derivatives of murrayanine‒chalcone were rationally designed and synthesized from murrayanine 1 and hydroxylated acetophenones 2A‒C to obtain hydroxylated derivatives using the previously reported protocol and to explore the anti‒inflammatory potential using carrageenan‒induced paw edema method. In addition to it, the establishment of the possible structure‒activity‒relationship (SAR) was done, which will be beneficial for the medicinal chemists in further designing target modulators.
Chemicals and instrumentation
The starting material ‘murrayanine’ was extracted from powdered M. koenigii stem bark by the soxhlation process as per our previously published extraction protocol. The analytical grade reagents and solvents were procured exclusively from Sigma Aldrich, Germany through a local vendor. The final synthesized compounds were characterized comprehensively by sophisticated spectroscopic techniques. The FT‒IR spectra were obtained using Shimadzu® IRAffinity‒1 instrument and the results were expressed in cm‒1. The 1H‒NMR spectra were acquired by utilizing Bruker Avance‒II instrument and calibrated with the internal standard, tetramethylsilane. The Mass spectra were achieved by employing MICROMASS Q‒TOF instrument. The CHN analyses of the synthesized derivatives were performed by means of PerkinElmer Elemental Analyzer 2400 instrument. The progress of the chemical reaction was inspected by utilizing pre‒coated Merck silica gel‒G TLC plates.
Animals
The anti‒inflammatory perspectives of synthesized hydroxylated derivatives were explored in albino rats (5‒6 weeks age, body weight 160‒270g) after obtaining approval from the CPCSEA (1389/a/10/CPCSEA) and Department Ethical Committee (DEC). The experimental rats were kept in the animal house under the hygienic condition with following protocol: two rats per cage, 25–26ºC temperature, humidity 50–65%, 1 hr light and dark, fed with standard rodent pellets, and provided free access to water.
Synthesis of target compounds
The novel molecules were synthesized from the active carbazole murrayanine (1) which was procured from the M. koenigii L. stem bark. The chalcone derivatives 3A‒C were fabricated by utilizing the aldehydic (‒CHO) portion of the murrayanine with the acetyl portion (‒COCH3) of acetophenone. Here, aldol condensation reaction took place where an enolate ion reacts with the carbonyl portion to liberate a β‒hydroxy ketone intermediate with further dehydrates to yield a conjugated enone. Scheme 1 illustrates the synthetic protocol. Equimolar (0.01M) quantity of murrayanine (1) and substituted acetophenones 2a‒c were taken in a round bottom flask and dissolved in 25mL of 90% ethanol and kept for stirring. An aqueous solution (20mL) of sodium hydroxide was added drop wise to the above content and the reaction was continued for the period of 3hrs. The reaction mixture was kept overnight and the content was added to the crushed ice (containing dilute HCl) on the next day to obtain the final compound. The product was further washed thoroughly, filtered under vacuum using Buchner’s funnel, and suitably recrystallized with water‒ethanol mixture Figure 1.
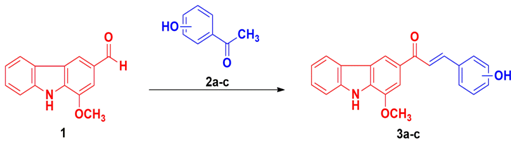
Figure 1The protocol for the synthesis of substituted hydroxylated murrayanine‒chalcone. Synthetic protocol for (E)‒3‒(substituted‒hydroxyphenyl)‒1‒(1‒methoxy‒9H‒carbazol‒3‒yl)prop‒2‒en‒1‒one 3(A‒C).
(E)‒3‒(2‒hydroxyphenyl)‒1‒(1‒methoxy‒9H‒carbazol‒3‒yl)prop‒2‒en‒1‒one (3a)
62% yield; FTIR (KBr) υ (cm‒1): 3427 (‒OH, stretching), 3306 (‒NH, stretching), 3085 (C‒H, aromatic), 1729 (C=O, stretching), 1632 (C=C, aromatic), 1601 (C=C, alkene), 1572 (‒NH, bending), 1297 (C‒N), 1222 (C‒O); 1H NMR (δ, ppm, CDCl3): 10.18 (9, 1H), 6.7‒8.1 (Aromatic, 10H), 5.35 (15, 1H), 3.84 (1, 3H). MS: M+ 343. Anal. Calcd. for C22H17NO3: C, 76.95; H, 4.99; N, 13.98. Found: C, 76.62; H, 4.57; N, 13.71
(E)‒3‒(3‒hydroxyphenyl)‒1‒(1‒methoxy‒9H‒carbazol‒3‒yl)prop‒2‒en‒1‒one (3b)
49% yield; FTIR (KBr) υ (cm‒1): 3411 (‒OH, stretching), 3286 (‒NH, stretching), 3107 (C‒H, aromatic), 1714 (C=O, stretching), 1619 (C=C, aromatic), 1595 (C=C, alkene), 1584 (‒NH, bending), 1277 (C‒N), 1203 (C‒O); 1H NMR (δ, ppm, CDCl3): 10.12 (9, 1H), 6.6‒8.4 (Aromatic, 10H), 5.33 (15, 1H), 3.91 (1, 3H). MS: M+ 343. Anal. Calcd. for C22H17NO3: C, 76.95; H, 4.99; N, 13.98. Found: C, 76.63; H, 4.44; N, 13.68
(E)‒3‒(4‒hydroxyphenyl)‒1‒(1‒methoxy‒9H‒carbazol‒3‒yl)prop‒2‒en‒1‒one (3c)
71% yield; FTIR (KBr) υ (cm‒1): 3423 (‒OH, stretching), 3246 (‒NH, stretching), 3159 (C‒H, aromatic), 1708 (C=O, stretching), 1644 (C=C, aromatic), 1606 (C=C, alkene), 1562 (‒NH, bending), 1281 (C‒N), 1241 (C‒O); 1H NMR (δ, ppm, CDCl3): 10.13 (9, 1H), 6.8‒8.3 (Aromatic, 10H), 5.29 (15, 1H), 3.89 (1, 3H). MS: M+ 343. Anal. Calcd. for C22H17NO3: C, 76.95; H, 4.99; N, 13.98. Found: C, 76.55; H, 4.49; N, 13.76
Acute toxicity studies
For the estimation of the in vivo safety profile, the OECD protocol was followed where the compound was administered at an increasing dose in the range of 25mg/kg to 500mg/kg. The exploration of acute toxicity study is essential since it reveals the dose where the highest therapeutic effect is achieved with no toxic symptoms. The safe dose was calculated based on the LD50 values.23
Anti‒inflammatory screening
The in vivo anti‒inflammatory activity of the fabricated chalcone derivatives 3A‒C was explored by carrageenan‒induced paw edema method. Here, the rats have initially fasted overnight in order to reduce the variation of the formed edema, since it affects the study significantly. Before the commencement of the study, 5mL of distilled water was administered to all the rats via the oral route. The inflammation was produced by injecting the 1% carrageenan solution in the right hind paw of the rat at the subplanter region. The molecules in the safest dose of 100mg/kg b.w. was initially suspended in the 0.9% saline solution and orally fed an hour prior to the instigation of the study. The control group was given 0.9% saline solution containing a few drops of Tween 80 (solubilizer). The thickness of the paw of the rats was determined using a mercury digital micrometer for the period of 3hrs following an interval of 1hr. The potentials of the molecule in reducing the edema were estimated by calculating the variation between the width of the injected paw and non‒injected paws. The results were expressed as mean±SEM.24
Statistical treatment
The results were analyzed by one‒way ANOVA method followed by the Dunnett’s multiple comparisons test. The P‒value <0.01 was considered statistically significant.
Chemistry
The sophisticated spectroscopic analysis revealed several facts that helped in the structural elucidation of the hydroxylated chalcone compounds. The FT‒IR spectra revealed some imperative facts that supported the formation of chalcones. The appearance of a broad peak of the hydroxyl group in the range of 3200‒3400cm‒1 along with a carbonyl function in the range of 1705‒1725cm‒1. In addition to it, the appearance of C=C (alkene) in the range of 1600‒1650cm‒1 represented that chalcones were formed from the starting material murrayanine. While looking at the proton‒NMR spectra, furthermore, the peak at 5.2‒5.35ppm signified the hydroxyl (‒OH) moiety in the compound (at position‒15). Moreover, the FT‒IR spectra showed that the murrayanine portion of the molecule had –NH stretching (at position‒9), ‒NH bending (at position‒9), and C‒O (at position‒1) part in the range of 3100‒3300cm‒1, 1575‒1650cm‒1, and 1200‒1300cm‒1, respectively, which supported the presence of murrayanine ring in the molecule. As well, the 1H‒NMR spectra supplementary supported the above facts, owing to the appearance of the peaks in the range 10.1‒10.2ppm and 3.8‒3.9ppm, respectively. The FT‒IR spectra presented some crucial aspects of the aromatic rings. The C=C (aromatic) and C‒H stretching were noticed primarily in the range of 1600‒1625cm‒1 and 3050‒3150cm‒1, respectively. The protons of the aromatic ring appeared in the range 6.6‒8.4ppm. The mass spectra expressed the emergence of base‒peaks of the molecules corresponds precisely with that of their theoretical molecular mass. Besides, a number of fragment peaks in the m/z range of 100‒200 were also seen in the mass spectra. The CHN analyses strongly supported the formation of the chalcone derivatives as indicated from the obtained practical values.
Acute toxicity study
All the three chalcone derivatives 3A‒C expressed no such toxic effect over the tested doses and were regarded as safe for the in vivo anti‒inflammatory study at 100mg/kg b.w.
Anti‒inflammatory activity
The fabricated molecules 3A‒C expressed moderate anti‒inflammatory activity in carrageenan‒induced paw edema model. The compound 3c having para substituted hydroxyl group exhibited noteworthy edema reducing potential of 35.76%, 41.18%, and 48.33%, respectively at 1hr, 2hrs, and 3hrs. The edema reducing activity may be due to the interaction of the substituents (of murrayanine‒chalcone) with the inflammatory targets like cyclooxygenase‒1/2 (COX‒1/2) and lipoxygenase (LOX). However, compounds 3a and 3b presented a lower anti‒inflammatory activity than that of the best active and the standard drug indomethacin. From this study, a SAR can be drawn where para substitution (3c) expressed the highest activity followed by meta (3b) and ortho (3a) substitution. The probable reason that 3c demonstrated better activity than the two other derivatives which may be supported by the “three‒point receptor theory”. Here, only one structural form possessed optimal spatial arrangement of the substituents (three‒groups) to interact with the active sites of the receptor to form either ionic, hydrophobic or hydrogen bond. The lack of achieving these interactions leads to reduced pharmacological activity because it will unable to interact and fit with the active site of the receptor and cannot trigger the action.25 On comparison of the activity with the previously synthesized heterocyclic analogs of murrayanine‒chalcone (pyrimidine13, oxadiazole6, thiadiazole8, and methylenedioxy16), it was noticed that the activity of the hydroxylated compounds was low. This study provided some clues for better optimization of the structure to achieve the pronounced biological activity Table 1.
Group |
‒R |
Percentage (%) inhibition of Edema |
||
|---|---|---|---|---|
1 hr |
2 hr |
3 hr |
||
3a |
2‒OH |
13.47±2.08 |
21.37±2.21 |
29.92±1.91 |
3b |
3‒OH |
21.84±2.14 |
33.62±2.16 |
40.56±1.66 |
3c |
4‒OH |
35.76±1.33 |
41.18±1.69 |
48.33±1.59 |
Indomethacin |
‒ |
43.23±1.98 |
52.02±1.41 |
66.39±1.78 |
Table 1 In vivo anti‒inflammatory activity of hydroxylated murrayanine chalcone derivatives
n = 6; ED50 of 100mg/kg b.w. in male adult albino mice; P<0.005
The present research study demonstrated the potentials of hydroxylated derivatives of murrayanine‒chalcone. The compound 3c having para substituted hydroxyl group exhibited noteworthy edema reducing potential. The compounds 3a and 3b presented a lower anti‒inflammatory activity than that of the best active and the standard drug indomethacin. The SAR expressed that the para substitution (3c) expressed highest biological activity followed by meta (3b) and ortho (3a) substitution. The exploration of acute toxicity revealed the safety profile of the compounds and all the compounds were tested at 100mg/Kg body weight. The edema reducing activity may be due to the interaction of the substituents (of murrayanine‒chalcone) with the inflammatory targets like cyclooxygenase‒1/2 (COX‒1/2) and lipoxygenase (LOX). The current study provided some imperative evidences for the better optimization of the chemical structure to attain marked biological activity.
Authors are highly thankful to Savitribai Phule Pune University, Pune, Maharashtra, India for providing research grants (Grant No. 13PHM000126).
Author declares that there is no conflict of interest.

©2018 Mahapatra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.