MOJ
eISSN: 2575-9094


Research Article Volume 2 Issue 3
1Department of Pharmacology, ASBASJS Memorial College of Pharmacy, India
2Department of Pharmaceutics, Indo-Soviet Friendship Colleges of Pharmacy, India
Correspondence: Ajay Singh Kushwah, Department of Pharmacology, ASBASJS Memorial College of Pharmacy, India, Tel +91-9417459195
Received: May 17, 2018 | Published: June 5, 2018
Citation: Saini K, Kushwah AS, Gupta GD. Soluble dietary fiber fraction of trigonella foenu graecum seeds prevent olanzapine induced metabolic syndrome in rats. MOJ Drug Des Develop Ther. 2018;2(3):116-123. DOI: 10.15406/mojddt.2018.02.00037
Trigonella foenum graecum (TF) belongs to the family Fabaceae, it has been from ancient time, used as a spice and traditionally ethno pharmacological medicine for promoting digestion and reduce blood sugar levels in a diabetic patient in India. In the present study, soluble dietary fiber (SDF) was isolated from TF seeds and investigates the role of SDF of TF, against olanzapine induced metabolic complications. Metabolic syndrome was induced by oral administration of OLZ 5mg/kg in rats for 28 days. Then rats were treated with OLZ and SDFF of TF at 250 and 500mg/kg; po./day and metformin as a standard 100mg/kg; po./day. SDF of TF treatment significantly decreased body weight gain, feed and water intake, blood glucose, oral glucose tolerance test (OGTT), lipid profile, liver enzymes, uric acid and improved hemodynamic parameters, antioxidant status and reduced histopathological changes caused by OLZ treatment in rats. SDF of TF increased the satiety, suppresses appetite and improved the glucose and lipid metabolism and antioxidant potential exert the beneficial role in olanzapine induced metabolic syndrome.
Keywords: olanzapine, metabolic syndrome, soluble dietary fiber, trigonella foenum graecum, galactomannan
MS, metabolic syndrome; AMPK, activated protein kinase; AGRP, agouti related peptide; BAT, brown adipose tissue; SDF, soluble dietary fiber
People with severe mental illness die up to 3 decades earlier than the general population and cardiovascular diseases are responsible for more than 50% of mortality in the population with schizophrenia.1 Average life expectancy in people with schizophrenia is about 20 years shorter than the normal population.2 It is reported that cardio metabolic complications such as obesity, hypertension, dyslipidemia, insulin resistance, diabetes mellitus associated with metabolic syndrome (MS) are well‒recognized side effects of antipsychotics particularly the atypical which are widely used today for schizophrenia.3 The introduction of atypical antipsychotics proves to be a significant advance in the treatment of schizophrenia as compared to older, typical antipsychotics provides some improvement in negative and cognitive symptoms, while exhibiting the marked reduction in extrapyramidal side effects.4 Cardiovascular side effects of antipsychotics may due to increase in appetite, weight gain, and hyperglycemia and lipid metabolism disturbances. Literature revealed that olanzapine leads to higher metabolic side effects than most other antipsychotics, only second to clozapine.5 The mechanisms underlying olanzapine (OLZ) induced weight gain and metabolic dysfunctions involve both central and peripheral mechanisms which converge to metabolic dysfunction.6 Initial increases in body weight are primarily caused by increases in appetite due to antagonism of multiple central receptors including 5‒HT2C, histamine H1 and dopamine D2.7 Weight gain and obesity associated with olanzapine are mediated by activation of the hypothalamic AMP‒activated protein kinase (AMPK) pathway via blockade of the H1 receptor.8 OLZ, in the arcuate nuclei (Arc) of the hypothalamus,down‒regulates the anorexigenic neuropeptide proopiomelanocortin (POMC) but up‒regulates the orexigenic neuropeptide Y (NPY) and agouti related peptide (AGRP). Furthermore, it also reduced activation of the brown adipose tissue (BAT) is associated with obesity and diabetes in humans.9 OLZ also change the levels of hormones related to weight regulation, such as insulin and leptin which may affect the sensitivity of satiety neurons in the lateral hypothalamus.10 It increases ghrelin level which is responsible for the increase in appetite and also associated with peroxisome proliferator‒activated receptors and transcriptional regulators of lipid and carbohydrates metabolism.11 Metabolic complications with atypical antipsychotics have raised concerns about a possible relation between these metabolic effects and treatment with these medications.12 Psychiatrists need to educate and motivate patients to make healthy lifestyle changes. If these lifestyle changes fail, these patients need to receive drug interventions. Adding medications such as metformin, topiramate, and amantadine or switching to another antipsychotic drug should be considered to decrease the risk of antipsychotic induced weight gain and metabolic abnormalities.13 In the battle to prevent obesity and related problems associated with olanzapine, satiety inducing food ingredients such as dietary fiber offer a natural dietary strategy for caloric intake control and body weight regulation.14 Dietary fiber could play an important role in the management of the metabolic complications and to positively affect those factors implicated in cardiovascular disease.15 The plant Trigonella foenum‒graecum (TF) belongs to family Fabaceae is the famous spice, it is commonly known as Methi (Hindi) and Fenugreek (English). TF is known for its pleasantly bitter and slightly sweet seeds.16 It is mostly cultivated in India, parts of West Asia, Middle East, North Africa, United Kingdom, Russia, Mediterranean Europe, Australia, US and Canada.17 Traditionally and ethno pharmacological uses of TF are in various disorders as antidiabetic, antihypercholesterolemic antioxidant, hepatoprotective, gastroprotective, antinociceptive, antibacterial, anti‒fungal, anti‒inflammatory and antineoplastic.18‒21 Phytoconstituents of Trigonella foenum‒graecum present in seeds such as carbohydrates, saponins, flavonoids, hemicelluloses, mucilage, tannins, 4‒hydroxyl‒isoleucine and pectin and especially fiber which is comprised of mainly 50% fiber (30% soluble fiber and 20% insoluble fiber).22 Also, TF husk is a valuable source of soluble dietary fiber (SDF) and phenolic acids; therefore, it could be an effective source of natural antioxidants.23 Galactomannans are the main soluble fiber present in seeds and form a gelatinous structure (similar to guar gum) which may have effects on slowing the digestion and absorption of food from the intestine and create a sense of fullness in the abdomen, thus suppresses appetite and promotes weight loss.24,25 The rationale of selection of the plant revealed that presence of various phytoconstituents which are responsible for the different activities. However, there are no sufficient studies have been carried out to explore the role of soluble dietary fiber rich fraction of TF, whereas a number of studies show that extract and powder of seeds ameliorate reduction in weight, glucose and lipid profile.26,27 Therefore, thought to work on SDF of TF seeds to explore its potential to reverse the metabolic complications associated with atypical antipsychotics. In the present study aims to investigate the role of SDF of TF in the prevention of olanzapine induced metabolic syndrome in rats.
Plant materials and preparation soluble diateryfraction (SDF) of Trigonella foenum‒graecum (TF)
Seeds of the plant were purchased from the local market and authenticated by Dr. Sunita Garg, Head of the Raw Materials Herbarium & Museum, Delhi (CSIR‒NISCAIR) Ref. No. NISCAIR/RHMD/Consult/2017/3088‒37. Trigonella foenum graecum (TF) seeds were dried at 40°C and finely powdered. The powder was weighed and 10% of the dilute acetic acid solution was added; mixed thoroughly with the mechanical stirrer for four hours and centrifuged using 3000rpm for 30min. The supernatant solution was transferred and volume was measured, four volumes of 95% ethyl alcohol were added, mixed thoroughly and allowed to settle for one hour to get precipitation of soluble dietary fiber (SDF) fraction. It was filtered through muslin cloth and washed with 95% ethyl alcohol and the residue was collected and dried overnight in hot air oven at 60°C and powdered.15 This isolated powder had 66.91% SDF enriched with galactomannan.28
Drugs preparation
Olanzapine procured from Micro Labs Ltd. Baddi (HP) India, was dissolved in normal saline and administered at the dose of 5mg/kg, po./day. Metformin was obtained as gift sample from Swaroop Drugs & Pharmaceuticals Maharashtra, India and dissolved in normal saline and administered at a dose level of 100mg/kg, po./day. All the other chemicals used were of analytical grade.
Experimental animals
The research protocol of this study had been approved by Institutional Animal Ethics Committee (IAEC) of ASBASJSM College of Pharmacy, Bela (Ropar) vide approval no. ASCB/IAEC/08/16/109.Thirty‒five albino female Wistar rats weighing 200‒220g were procured from Central Animal Facility, AIIMS, New Delhi. The animals were kept in quarantine section till monitoring of health status of animals and subsequently transferred to the housing area. The animals were housed in standard size polypropylene cages using husk as bedding in the controlled conditions of temperature (23±2°C), humidity (40±10%) and 12:12h dark/light cycles. The animals were fed with standard diet and purified water was given ad labium as per CPCSEA guideline.
Experimental design
The Female Wistar rats of weight 200‒220gm were divided into six experimental groups (n=5‒6/group) and were matched by body weight. The following six groups were studied for 28 days Figure 1. Normal control (C) rats received only 0.1ml normal saline, SDF of TF parse group (TF 500) rats were received SDF 500mg/kg; po./days, Olanzapine (OLZ) group administered 5mg/kg; po./days of olanzapine to induce metabolic syndrome and study group OLZ with two different dose of SDF of TF at dose 250/500mg/kg; po./days(OLZ+TF 250) and (OLZ+TF 500) respectively; OLZ with Metformin standard group (OLZ+Metformin) 100mg/kg/po/days. The standard and treatment drugs were administered after OLZ treatment. The doses were selected on the basis of literature report.19,29 The body weight, food intake and water intake of rats were evaluated daily. Blood analysis and hemodynamic parameters evaluated after the end of the study 29th day. After these studies, the animals were sacrificed by decapitation to estimate tissues TBARS, GSH levels, and histopathological studies were carried out.
Serum biochemical analysis
The blood sample was obtained from the overnight (16h) fasted rats after the administration of the last dose (on 29th day). The blood was collected by puncturing retro‒orbital plexus using heparinized glass capillary tubes under chloroform anesthesia and collected in Eppendorf tubes and then centrifuged at 3000rpm (40°C 10min) serum was separated for analysis of different haematological parameters such as Glucose, OGTT, cholesterol, HDL (high density lipoprotein), LDL (low‒density lipoprotein), TG (triglycerides), SGOT and SGPT and uric acid. All analysis was performed with‒commercially available kits using autoanalyzer (Reckon Diagnostics, Chandigarh and Span Diagnostics Ltd., Surat, Erba Diagnostics, Baddi). TBARS, GSH levels, and histopathological studies were carried out.
Oral glucose tolerance test (OGTT)
OGTT was performed by measuring plasma glucose at the end of the study period in each group. The rats fasted for 16h and blood samples were collected by retro‒orbital plexus. A dose of 2g/kg (body weight) glucose solution was given by gastric gavages. Blood samples were obtained from the retro‒orbital plexus at pre and 30, 60 and 120min. post glucose intake. Plasma glucose levels were measured by available kits using autoanalyzer (Reckon Diagnostics, Chandigarh).
Measurement of hemodynamic parameters
Rats were anesthetized with 25% urethane (1.5g/kg, i.p). Throughout the experimental protocol body, temp of the animals was maintained at 37ºC. The neck was opened with a ventral midline incision to perform the tracheotomy. The left carotid artery was cannulated with the Polyethylene tube (internal diameter 0.30mm; outer diameter 0.40mm) attached to a three‒way cannula. The cannula was heparinized (Heparin 300IU/ml) and connected to POWER LAB 4/30 (AD Instruments, NSW, Australia) system using a pressure transducer for the measurement of systolic, diastolic, mean arterial pressures and heart rate.
Measurement of biochemical parameters
Animals were sacrificed by cervical dislocation and the heart tissues were removed, washed with the cold isotonic saline and dried with filter paper. After this hearts were isolated and homogenize in 0.05M ice cold phosphate buffer. After centrifugation, the supernatant was used for analysis of antioxidant enzymes GSH and TBARS.30,31
Histopathology
The animals were sacrificed; the liver was isolated, washed with ice cold saline and fixed in neutral buffered formalin (NBF) for not less than 6 hours.32 The samples were sent pathology department of IVY hospital, sec‒71, S.A.S Nagar (Mohali) for the preparation of slides of liver tissue and stained using H & E staining technique. For interpretation, the pictures were taken from the prepared slides with the help of photo‒ microscope.
Statistical analysis
The data were expressed as Mean±SEM was analyzed by one‒way ANOVA followed by Tukey multiple comparison tests using Graph Pad Prism software package. A value of P<0.05was considered to be significant.
Effect of soluble diatery fraction (SDF) of Trigonella foenum‒graecum (TF) on body weight, feed intake and water intake
Antipsychotic‒induced metabolic disorders are the risk factors for cardiovascular disease, insulin resistance and diabetes mellitus resulting in increased morbidity and mortality.33 Weight gain has been very serious issue frequently with olanzapine and other atypical antipsychotics drugs.34 Several mechanisms of olanzapine have been postulated, including its antagonistic effect on D2, 5‒HT2C, H1 and M3 receptors, some reports have correlated the weight gain with leptin and/or ghrelin, which cause hyperphagia.34 Various scientific reported that olanzapine administration could induce weight gain in the female, but not male rats.35 Some natural drugs containing phenolic and flavonoids have reversal effects on metabolic complications associated with olanzapine.36 Numerous therapies found that metformin can improve weight gain and insulin resistance induced by antipsychotics and reduce metabolic syndrome or type‒2 diabetes.37 It is reported that effect of TF mediated by the ability of galactomannan to reduce absorption of lipids by inhibition of intestinal lipases enzyme, decreased HMG‒CoA reductase activity and increased biliary cholesterol excretions.38 OLZ is reported to increase in weight gain, feed and water intake in several clinical and animal studies.39 In study elucidated the effects of SDF of TF supplementation on OLZ (5mg/kg, po.) induced metabolic syndrome. Body weight, feed, and water intake were compared with normal control rats; OLZ group and OLZ with TF (250/500mg/kg) and metformin group, the initial body weights were not different among the groups. In study animals treated with olanzapine increased body weight, feed and water intake as compared with normal control group (Table 1) (Figure 2) (Figure 3). The body weight gain in OLZ groups was higher than in the normal control group after the 28th day. Whereas the body weight of OLZ with SDF of TF at 250 and 500mg/kg and OLZ with metformin group were observed to significantly decrease body weight, feed and water intake compared to the OLZ group (P <0.05). The high dose of TF 500 showed more significantly (P<0.05) results as compare to lower dose of TF 250. An increase in feed consumption indicates that OLZ causes hyperplasia and leads to weight gain in rats.29 Body weight was found to be significantly elevated, probably due to increased feeding. Some studies show that increased feed efficiency and insulin resistance and activated hypothalamic AMPK are also might be involved in OLZ induced weight gain.40 It has been proposed that olanzapine leads to an alteration in adipocytokines such as leptin which regulate the body weight by an effect on food intake, energy consumption and glycolipid metabolism.41
Groups |
Average weight gain |
Percentage change |
Normal control |
25±2.069 |
115±1.372 |
TF (500mg/kg) |
17.58±2.227 |
110.0±1.377 |
OLZ control (5mg/kg) |
90.8±1.782a |
154.0±1.428a |
OLZ+TF (250mg/kg) |
81.4±2,42b |
149.0±2.035b |
OLZ+TF (500mg/kg) |
56.07±1.556b,c |
132.8±1.074b,c |
OLZ+Metformin (100mg/kg) |
48±1.572b |
128.2±0.8881b |
Table 1 Effect of soluble diateryfraction (SDF) of trigonella foenum-graecum (TF) on average weight gain and average percentage change of body weight
The total duration of the study was 28 days.
Values are expressed as Mean±SEM (n=5‒6). a(p< 0.05) vs normal control group;
b(p< 0.05) vs olanzapine control group; c(p< 0.05) vs low dose TF (250mg/kg) treatment group
(One way ANOVA followed by Tukey’s test)
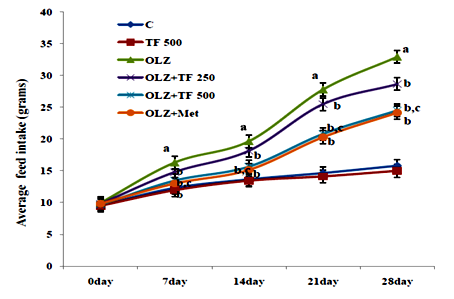
Figure 2 Effect of soluble dietary fiber fraction (SDF) of trigonella foenum graecum seeds (TF) on feed intake.
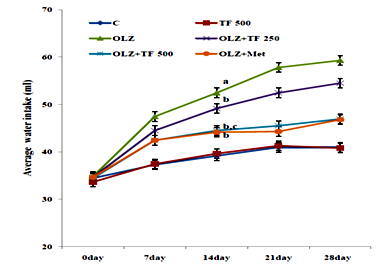
Figure 3 Effect of soluble dietary fiber fraction (SDF) of trigonella foenum graecum seeds (TF) on water intake.
Effect of soluble diateryfraction (SDF) of trigonella foenum‒graecum (TF) on serum biochemical parameters
It has been reported also that TF acts by delaying glucose absorption and enhancing its utilization and hypoglycemic effect of TF are due to phytoconstituents mainly galactomannan present in seeds which help in the management of metabolic abnormalities associated with diabetes such as peripheral insulin resistance and lipid abnormalities.38 Anti platelet activity PPARγ agonists and HMG‒CoA reductase inhibitors like constituents in this plant exerts beneficial effects on several physiologic markers relevant to metabolic syndrome. Olanzapine increased the liver fat and enzymes thus promote the intrahepatocellular lipids and cause endothelial dysfunction.42 TF fiber has been shown to inhibit glucose absorption and decrease postprandial hyperglycemia and these effects were attributed to delay gastric emptying of carbohydrate, inhibition of intestinal lipase and increased gut motility. It has been proposed that TF seeds exert hypoglycemic effects by stimulating glucose dependent insulin secretion from pancreatic beta cells. In the present experiment, the continuous treatment for 28days with the TF showed a potential anti hyperglycemic effect and OGTT activity when compared to OLZ treated rats. Studies show that OLZ induced glucose and insulin dysregulation may be partly due to blockade of muscarinic M3 receptors, causing disruption to insulin secretion and glucose homeostasis that can progressively lead to insulin resistance and diabetes during chronic treatment.43 The ability to modify glucose homeostasis perhaps due to the presence of β‒glucan and fiber fraction increased both insulin‒stimulation and basal glucose uptake by AMPK activation as reported in previous.44 Long‒term consumption of olanzapine produces a lipoprotein profile, associated with the development of the cardiovascular disorder and metabolic syndrome. Elevated plasma concentrations of total cholesterol (TC), low‒density lipoprotein (LDL) cholesterol and reduced high‒density lipoprotein (HDL) are commonly seen in dyslipidemia. The administration of olanzapine results in excess hepatic lipids accumulation due to increased synthesis and decreased secretion of lipids and increased lipogenesis.45 OLZ group in the plasma biochemical analysis glucose, cholesterol, triglycerides (TG), LDL, SGOT, SGPT and uric acid levels increase significantly (P<0.05) were as significantly lower HDL level as comparing the normal (P<0.05). Effects of TF (SDF) on the biochemical parameters of glucose and lipid levels in the OLZ+TF 250 and OLZ+TF 500 group had significantly (P<0.05) reduced cholesterol, TG, and LDL levels, whereas HDL levels in OLZ with TF groups increased when compared to the OLZ group. In OLZ+ Metformin group Glucose, cholesterol, TG, LDL levels significantly (P<0.05) decreased and HDL significantly (P<0.05) increased when compared to OLZ control group Table 2. It is reported that hypolipidemic effect of TF mediated by the ability of galactomannan to reduce absorption of lipids by inhibition of intestinal lipases enzyme and demonstrated anti‒atherogenic effects via decreased serum LDL oxidation, increased biliary cholesterol excretions.46‒47 SGOT and SGPT enzymes are a sensitive marker of liver damage and these enzymes levels are predictive of damage to the liver. Hence any necrosis or membrane damage to the liver leads to leakage of these enzymes into the blood circulation. In our findings significantly increase (P<0.05) of the level the diagnostic marker such as SGOT and SGPT respectively in OLZ group, when compared to normal control group. Effects of TF (SDF) on SGOT, SGPT, and uric acid were significantly reduced in the OLZ with TF 250 and 500 groups when compared to the OLZ group (P<0.05). OLZ+ Metformin treated group SGOT and SGPT decreased significantly (P<0.05) and uric acid reduced non‒significantly in and when compare to OLZ treated rats. The reduced activities of the serum biomarker enzymes and enzymatic leakage in the liver may be due to enhanced antioxidant potential of TF seeds which could be responsible for attenuating reactive oxygen species induced oxidative stress and inflammation.48 It has been hypothesized that uric acid overproduction can trigger oxidative stress xanthine oxidoreductase (XO), the enzyme responsible for urate formation, may play a critical role in hyperuricemia and reduce the activity of nitric oxide synthase in metabolic syndrome.49 In studies of rats and humans, TF improved parameters associated with renal dysfunction due to the presence of antioxidant properties.50 Many investigations have confirmed the association of hyperuricemia with metabolic syndrome in antipsychotic patients. Uric acid may simply be a consequence of the increased uric acid absorption in the proximal tubule secondary to hyperinsulinemia there is growing data that uric acid may predict the development of metabolic syndrome, obesity, and diabetes.51 In our study in OLZ group, uric acid increased and TF (250, 500mg/kg) reduced compared with normal control rats. But metformin did not significantly reduce the uric acid level Table 2.
Parameters |
Normal Control |
TF 500 |
OLZ |
OLZ+TF250 |
OLZ+TF 500 |
OLZ+METFORMIN |
Glucose (mg/dL) |
96.68±1.952 |
93.53±2.788 |
182.8±2.536a |
158.6±2.066b |
120.4±0.733b,c |
119.9±1.845b |
Cholesterol (mg/dL) |
158.9±2.934 |
158.00±3.448 |
230.60±3.448 |
214.10±2.335b |
188.7±3.285b,c |
183.45±1.198b |
HDL (mg/dL) |
56.21±1.452 |
57.73±0.666 |
28.37±0.668a |
35.53±0.965b |
44.87±1.011 b,c |
45.22±0.5167b |
LDL (mg/dL) |
77.87±2.128 |
74.47±3.401 |
167.60±3.830a |
148.80±2.334b |
117.15±3.337 b,c |
109.6±0.7571b |
TG (mg/dL) |
124.1±2.422 |
129.1±1.932 |
160.2±1.782a |
148.7±1.034 |
133.4±0.810 b,c |
129.4±0.952b |
SGOT (IU/L) |
25.23±1.449 |
23.98±1.495 |
71.90±1.552a |
59.16±1.168b |
36.77±0.9236 b,c |
44.23±0.8597b |
SGPT (IU/L) |
33.13±1.203 |
33.46±1.138 |
81.09±1.771a |
63.66±0.6415b |
44.77±1.232 b,c |
54.32±0.4791b |
Uric Acid |
1.747±0.114 |
1.573±0.147 |
4.105±0.155a |
3.502±0.064b |
2.767±0.1516 b,c |
3.772±0.171 |
Table 2 Effect of soluble diateryfraction (SDF) of trigonella foenum-graecum (TF) on serum biochemical parameters
The total duration of the study was 28 days.
Values are expressed as Mean±SEM (n=5‒6). a(p< 0.05) vs normal control group;
b(p< 0.05) vs olanzapine control group; c(p< 0.05) vs low dose TF (250mg/kg) treatment group
(One way ANOVA followed by Tukey’s test)
Effect of soluble diatery fraction (SDF) of trigonella foenum‒graecum (TF) on oral glucose tolerace test (OGTT)
Oral blood glucose concentrations were determined immediately after the rats fasted overnight. The OGTT results for olanzapine group rats at 0, 30, 60 and 120min after solution ingestion are significantly increased (P<0.05) as compared to control groups. Study groups OLZ+TF 250/500 and OLZ+ Metformin group glucose level significantly reduced when compared to OLZ group (P<0.05). OLZ+TF (500mg/kg) showed significant decrease in glucose level at different time intervals compared to OLZ+TF (250mg/kg), (P<0.05) Figure 4. Studies show that OLZ induced glucose and insulin dysregulation may be partly due to blockade of muscarinic M3 receptors, causing disruption to insulin secretion and glucose homeostasis that can progressively lead to insulin resistance and diabetes during chronic treatment. The ability to modify glucose homeostasis perhaps due to the presence of β‒glucan and fiber fraction increased both insulin‒stimulation and basal glucose uptake by AMPK activation as reported in previous studies.
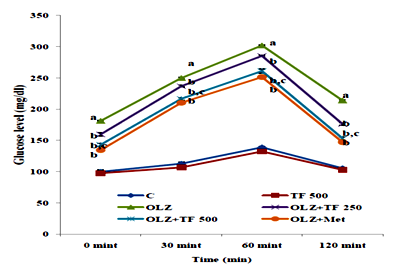
Figure 4 Effect of soluble diateryfraction (SDF) of trigonella foenum-graecum (TF) on Oral glucose tolerance test (OGTT).
Effect of soluble diateryfraction (SDF) of trigonella foenum‒graecum (TF) on hemodynamic parameters
OLZ showed the significant reduction in the AP, MAP but elevation in the SAP, DAP, and HR in the heart was observed when compared to normal control group. Oral administration of metformin (100mg/kg) show significant elevation (P<0.05) in the AP, MAP but a reduction in SAP, DAP, and HR in the heart when compared to olanzapine group. Treatment with TF (250 & 500mg/kg) shows significant elevation in the AP, MAP respectively (P<0.05), but the reduction in HR, SAP, and DAP with respect of dose when compared with olanzapine group.TF (500mg/kg) showed more significantly results when compared to TF (250mg/kg) (P<0.05) Table 3. Further evidence for the cardioprotective effects of TF has emerged in cardiovascular disease including hypertension due to vasodilatory properties and also protects vascular function by increasing the expression of endothelial NO synthase.52,53 Chronic treatment with olanzapine elevates systolic blood pressure in normal rats and weight gain is associated with elevation of diastolic blood pressure and it stimulates sympathetic nervous system activity which may elevate blood pressure.54 In the present study, administration of olanzapine shows a significant reduction in the AP, MAP but elevation in the SAP, DAP, and HR in the heart was observed when compared to normal control group. Oral administration of TF (250 & 500mg/kg) and metformin (100mg/kg) show a significant elevation in the AP, MAP but a reduction in SAP, DAP, and HR in the heart, when compared to OLZ group. The association between obesity and hypertension is well established in many studies.55 These observations are indicative of olanzapine induced weight gain playing a role in blood pressure elevation.
Group |
AP(mmHg) |
HR(BPM) |
MAP(mmHg) |
SAP(mmHg) |
DAP(mmHg) |
Normal |
118.7±0.921 |
362.9±1.722 |
123±1.446 |
120±1.253 |
84.6±1.21 |
Normal+TF (500mg/kg) |
119.7±0.710 |
367.3±1.786 |
120.8±1.482 |
121.6±1.414 |
84.1±0.781 |
OLZ control (5mg/kg) |
96.4±0.611a |
419.4±1.983a |
93.72±0.827a |
144.4±1.429a |
103.6±1.241a |
OLZ+TF (250mg/kg |
104.3±0.725b |
407.2±1.146b |
101.9±1.116b |
129.3±0.335b |
97.8±0.427b |
OLZ+TF (500mg/kg) |
111.8±0.943b,c |
396.1±0.790b,c |
112.7±1.107b,c |
124.1±0.643b,c |
92.6±0.427b,c |
OLZ+Metformin (100mg/k) |
106.3±1.062b |
399.3±0.526b |
108.6±0.778b |
128.8±0.742b |
95.2±0.728b |
Table 3 Effect of soluble diateryfraction (SDF) of trigonella foenum-graecum (TF) on hemodynamic parameters
The total duration of the study was 28 days.
Values are expressed as Mean±SEM (n=5‒6). a(p<0.05) vs normal control group; b(p<0.05) vs olanzapine control group; c(p<0.05) vs low dose TF (250mg/kg) treatment group
(One way ANOVA followed by Tukey’s test)
Effect of soluble diateryfraction (SDF) of trigonella foenum‒graecum (TF) cardiac TBARS and GSH
The effect on TBARS and GSH of TF (250 and 500mg/kg) is depicted in Table 4 statistical analysis revealed that there was significant difference among the groups, It revealed that OLZ group showed significant (P<0.05) elevation in TBARS levels and reduction (P<0.05) in GSH levels compared to control. Study group OLZ+ Metformin and TF (250 and 500mg/kg) showed significant (P<0.05) decrease in TBARS and increase (P<0.05) GSH level compared to OLZ group. TF (500mg/kg) showed significant (P<0.05) decrease in TBARS and increased (P<0.05) GSH levels compared to TF (250mg/kg) (P<0.05). Abnormalities in lipid metabolism decrease the strength of the ant oxidative defenses. Oxidative stress defined as a disruption of the delicate balance between oxidative and antioxidative process, play an important role in the pathogenesis of hypercholesterolemic atherogenesis. Oxygen free radicals (OFRs) are found to be produced during obesity and adiposity.56 GSH has a direct antioxidant function by reacting with super oxide radicals and singlet oxygen followed by the formation of oxidized GSH. It plays an important role in the regulation of a variety of cell functions and in cell protection against oxidative injury. The antioxidant of TF may be due to its free radical scavenging activity which neutralizes the free radicals generated during oxidative stress. MDA is the stress marker that indicates lipidperoxidation of endogenous lipid has been shown to be a major risk factor in metabolic syndrome. Free radicals produced by endothelial injury could initiate peroxidation of membrane‒ bound polyunsaturated fatty acids, leading to myocardial injury.57 In the present investigation, administration of olanzapine increased the level of MDA when compared to normal control group. The correlation found between dyslipidemia and oxidative stress in this study shows that dyslipidemia induced by ingestion of OLZ is the primary cause of lipid peroxidation. Therefore, the possible reason for the improvement in dyslipidemia with TF may be due to the reduction in oxidative stress in OLZ treated rats by improving lipid peroxidation and antioxidant enzyme status.58
Group |
MDA(µmol/ml) |
GSH(µmol/ml) |
Normal |
50.7±1.225 |
138.7±1.076 |
TF (500mg/kg) |
49.71±0.853 |
138.9±1.351 |
OLZ control (5mg/kg) |
82.26±1.022a |
55.66±1.721a |
OLZ+TF (250mg/kg) |
67.46±0.801b |
73.46±0.367b |
OLZ+TF (500mg/kg) |
54.6±1.154b,c |
95.05±1.027b,c |
OLZ+Metformin (100mg/kg) |
60.24±1.055b |
82.33±1.183b |
Table 4 Effect of soluble diateryfraction (SDF) of trigonella foenum-graecum (TF) cardiac lipid peroxidation (TBARS and GSH)
The total duration of the study was 28 days.
Values are expressed as Mean±SEM (n=5‒6). a(p<0.05) vs normal control group; b(p<0.05) vs olanzapine control group; c(p<0.05) vs low dose TF (250mg/kg) treatment group
(One way ANOVA followed by Tukey’s test)
Effect of soluble diatery fraction (SDF) of trigonella foenum‒graecum (TF) on histopathological changes in liver
In addition to the previous hypothesis, olanzapine causes direct damage to the liver and oxidative stress that is reflected by derangement of hepatic cells using H&E staining.59 The histopathological examination of the normal group showed normal cell architecture of the liver hepatocytes and no congestion of sinusoids. Hepatocytes were arranged in a network around the central vein while olanzapine group showed significant morphological changes such as degeneration, necrosis of hepatocytes in the liver cytoplasm and inflammation more, edema and congestion of blood vessels in the central vein. In metformin and SDF of TF, treated rats showed, considerably mild degenerations, less necrotic changes, congestion of blood vessels and edema and lesions were localized in some liver lobules in form of degenerative cell foci with fewer cells as compared to olanzapine treated rats Figure 5. Therefore, the present study suggests a protective effect of soluble dietary fiber rich fraction of Trigonella foenum‒graecum on olanzapine induced metabolic syndrome in rats. Such antioxidant properties of the SDF fraction of TF seeds may be valuable in the prevention and possible reversal of metabolic complications. The increase in satiety may be due to the presence of galactomannan in the SDF fraction of TF and due to its viscous gel forming property, is effective in inhibiting the intestinal glucose uptake and suppress appetite in rats. Comparative study of Metformin also indicates that treatment with SDF rich fraction of TF improves the lipid profile, cardiac marker, antioxidant status, hemodynamic and histopathological alterations in olanzapine treated rats. The protective effect of TF may be related to its free radical scavenging and helpful in protection from the metabolic disorders. Overall, these results signify the importance of Trigonella foenum graceum, a novel source of dietary fiber in the management of antipsychotic induced metabolic complications.
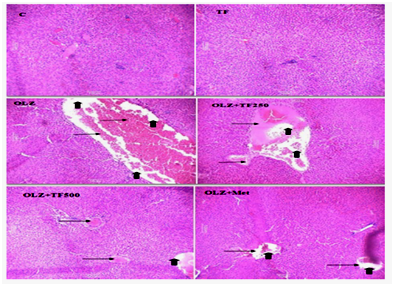
Figure 5 Effect of soluble diateryfraction (SDF) of trigonella foenum-graecum (TF) on hepatic histopathological changes in OLZ induced metabolic syndrome.
In normal control (C) and TF control, showed the normal histological appearance of the liver hepatocytes and central vein. Hepatocytes were arranged in a network around the central vein. In OLZ group (arrows) showed, edema and necrosis of hepatocytes in the liver cytoplasm. OLZ+TF (250mg/kg) showed less edema and necrotic changes. In OLZ+TF (500mg/kg) and OLZ+ Metformin showed, considerably mild hepatic changes when compared to OLZ group and lesions were localized in some liver lobules in form of degenerative cell foci with fewer cells.
Based on the results of the present study it was concluded that soluble dietary fiber rich fraction of Trigonella foenum‒graecum exert beneficially and of a promising role in olanzapine induced metabolic syndrome. These effects may have vital clinical importance and may be further improved, enhanced and should continue to be extensively studied.
Authors are thankful to the management committee of ASBASJS Memorial College of Pharmacy BELA (Ropar) for providing facilities to conduct the project.
Author declares that there is no conflict of interest.

©2018 Saini, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.