MOJ
eISSN: 2374-6912


Research Article Volume 4 Issue 2
Research and Development Department, Tokyo Metropolitan Industrial Technology Research Institute, Japan
Correspondence: Research and Development Department, Tokyo Metropolitan Industrial Technology Research Institute, Japan, Tel (+81)355302111, Fax (+81)355302671
Received: March 20, 2017 | Published: April 6, 2017
Citation: Monkawa A, Gessei T, Hachiya N. Existence of unprocessed a mitochondrial enzyme: YDL178wp in the membrane fraction as an oligomeric formation with a protein-unfolding activity. MOJ Cell Sci Rep. 2017;4(2):39-43. DOI: 10.15406/mojcsr.2017.04.00082
Gene product of the S.cerevisiae YDL178w (YDL178wp) has been identified as three proteins; firstly, it found as an actin interacting protein 2 (AIP2) using yeast two hybrid system, then re-named D-lactate dehydrogenase 2 (Dld2) because of its enzymatic activity and mitochondrial localization using fluorescent microscopy observation with the GFP-tag at the C-terminal of the protein. Finally, we have designated it Unfoldin, since it represented robust protein-unfolding activity with the Oligomeric formation. Here we show that YDL178wp exists as the two types of molecules in yeast cells, a mature sized mitochondrial protein and a precursor sized protein in the post-mitochondrial membrane fraction. This atypical intracellular presentation with multifunctional activity exhibits gene sharing or protein moonlighting, which would be represented the pragmatism of the evolutionary process.
Keywords:protein-unfolding, protein moonlighting, mitochondria, membrane, yeast
Unfold in, a Saccharomyces cerevisiae gene product of YDL178w (YDL178wp) formed a homo-oligomeric complex consisting of 10 to 12 subunits arranged in a horseshoe-like structure with the ~10 nm in diameter and ~2 nm of central cavity.1 It has a chaperone-like protein-unfolding activity; aggregated protein substrates such as amyloid b(1-42) or a-synuclein, increases its trypsin susceptibility even under the treatment of low concentration of the protease,2 indicating that Unfold in possesses the robust protein-unfolding activity.3,4 Adenosine triphosphate (ATP), but not its hydrolysis, promoted the binding of Unfoldin to protein substrates without any specificity in vitro,5 and the removal of its C-terminal coiled-coil region led to the dissociation of oligomer concomitant with the loss of both substrate-binding and protein-unfolding activities.1,2 Electron microscopy observation revealed that Unfoldin adapted at least in two states that correspond to either an “open state” in the presence of ATP or a “closed state” in the absence of ATP. Monomeric protein which consists of Unfoldin,1 YDL178wp, was initially identified as an actin interacting protein 2 (AIP2) using a yeast two-hybrid system,6 and then it was renamed as D-lactate dehydrogenase 2 (Dld2),7 because of the homology of Dld1p though the mitochondrial enzymatic activity is exhibited only weakly.
Here we show that YDL178wp exists two types of molecules in yeast cells: 59 KDa of L-form and 55 KDa of S-form. Unfoldin; the cytosolic oligomeric form of the protein with the protein-unfolding activity consisted only of the L-form, which has an unprocessed mitochondrial 36 amino acids as a mitochondrial presequence at the N-terminal. Meanwhile, S-form exhibited smaller sized molecule as a mitochondrial mature protein, which is processed mitochondrial presequence region at the site of N-terminal amino acids Y36 and S37. These results exhibited that YDL178wp belongs to the proteins known as “protein moonlighting”; one gene product has various functions with alternative localization.8,9
Protein-conformation modifying assay
The assay was performed as described before.10 Briefly, to prepare for the assay substrate, the gene of yeast pheromone pro-alpha factor (pαF) was added hexahistidine-tag at the C-terminal and amplified by PCR then inserted into the E. coli expression vector pET11a (Novagen). To express the protein, the plasmid was transformed into E.coli BL21 (DE3). The expressed recombinant protein was suspended in 20 mM of NaPi pH 8.0 containing 7 M of urea, then purified using Ni-NTA agarose column (QIAGEN). Bounded samples were eluted with 500 mM of imidazole buffer, following dialysis in 20 mM of NaPi pH 7.0 containing 150 mM of NaCl. The assay was started by adding 0.1 mg of pαF to 200 μl of buffer A (10 mM of HEPES-KOH pH of 7.4, 1 mM of DTT, and 1 mM of Mg(OAc)2) containing 1 mM of ATP. The mixture was incubated at 30°C for 15 min then digested with trypsin (200 ng/ml) at 16°C ° for 15 min. Trypsin digestion was stopped by incubation with soybean trypsin inhibitor (400 ng/ml) on ice for 5 min, followed by trichloroacetic acid (TCA) precipitation with tRNA carriers. The untrypsinized and protected pαF was evaluated by SDS-PAGE and Western blotting using affinity-purified polyclonal rabbit anti-pαF antibody as the first antibody and horseradish peroxides-linked IgG (ICN Inc, Cappel Products) as the secondary antibody. The immunoreactivity bands were visualized by ECL-prime (GE Healthcare Science) and analyzed using a Versa D°C 5000 System (Bio-Rad).
Purification of hexahistidine-tagged Unfoldin
To obtain sufficient amounts of Unfoldin, we prepared the yeast strain expressing YDL178w under the control of ADH promoter. The gene was fused at the C-terminal with hexahistidine-tag then amplified by PCR and then inserted into aureobasidine A (AbA) selective expression vector pAUR12311 (Takara Bio Chemicals). The protease-deficient yeast strain BY2778 [MATα prb1 -D1.6R pep4::HIS3 ura-3-52 his3-D200 can1 gal2] was transformed with this plasmid, grown in YPD medium containing 0.5 mg/ml of AbA at 30°C. Cells were collected and treated with 2.5 mg/ml of zymolyase 20T then homogenized using glass pestle downs homogenizer. The debris was removed by centrifugation at 3,000-x g for 5 min; supernatants were centrifuged at 12,000-x g at 4 °C for 8 min. This pellet was used as the mitochondrial fraction. Post-mitochondrial supernatants were ultra-centrifuged at 100,000-x g at 4 °C for 1 h, this precipitates were used as post-mitochondrial membrane fraction. Both samples were resuspended in buffer B (50 mM of NaPi pH of 8.0, 150 mM of NaCl, and 10 mM of imidazole), subjected to Ni-NTA agarose column (QIAGEN, K.K.) equilibrated with buffer B and eluted with 500 mM of imidazole-containing buffer B. The elute was dialyzed against buffer C (10 mM of HEPES-KOH pH of 7.4, 50 mM of NaCl, and 1 mM of DTT), passed through DEAE column (GE healthcare) equilibrated with buffer C and eluted with 500 mM of NaCl, followed by the separation with the gel filtration chromatography on a Superdex 200 column (GE healthcare) equilibrated with buffer D (50 mM of NaPi pH of 7.5, 10 mM of NaCl, and 1 mM of Mg (OAc)2). Purified samples were evaluated by SDS-PAGE and Western blotting using anti-histidine antibody (Cell Signaling Technology) as the first antibody and horseradish peroxides-linked IgG (ICN Inc, Cappel Products) as the secondary antibody. The immunoreactivity bands were visualized by ECL-prime (GE Healthcare Science) and analyzed using a Versa Doc 5000 System (Bio-Rad).
Proteinase K digestion of ScN2a cells
For the treatment of ScN2a cells with the L- and S-form of YDL178wp, the cells were collected at the 80% confluent then suspended in the 2 volume of PBS. Cell suspension (1 mg/ml) was crushed with grass beads and the incubated with 0.1 mg/ml of the L- or S-form of samples in10 mM of HEPES-KOH pH of 7.4, 1 mM of DTT, and 1 mM of Mg(OAc)2 containing 1 mM of ATP at 30°C for 1h. After this incubation, cells were resuspended in the lysis buffer (50 mM of Tris-Cl pH of 7.8, 150 mM of NaCl, 0.5% sodium deoxycholate, and 0.5% NP-40) then 10 mg/ml of proteinase K was added. After the incubation at 37°C for 30 min, cells were centrifuged with 10,000-x g and the pellet was collected and analyzed via SDS-PAGE and Western blotting using anti-prion protein monoclonal antibody SAF83 (Cayman Chemicals).
The C-terminal hexahistidine-tagged YDL178w was expressed in the protease-deficient yeast strain BY2778 under the control of ADH-promoter using aureobasidine A (AbA) selective expression vector pAUR123. Cells were grown in YPD medium containing 0.5mg/ml of AbA11 at 30°C. Each samples treated with zymolyase 20T, then cells were homogenized and fractionated into mitochondrial12 or post-mitochondrial fractions by using the centrifugation method as described in Methods section; the latter was usually used as the starting material for Unfold in preparation (Figure 1). In these experiments, mitochondrial and post-mitochondrial samples were firstly examined using two column chromatography steps, Ni-NTA and ion exchange chromatography, followed by gel filtration chromatography, respectively. From the mitochondrial fraction-derived materials, the eluted peak fraction of the gel filtration was observed at the fraction of 19, indicating at the size of ~ 55 KDa monomeric sizes (Figure 2A). In contrast, the peak fraction from post-mitochondrial fraction-derived sample was detected at the size of ~700 KDa, indicating the oligomeric form (Figure 2B).
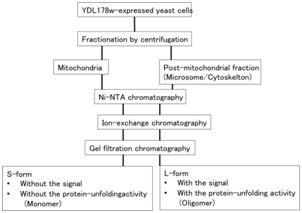
Figure 1 Purification scheme of YDL178wp/Aip2p/Dld2p/Unfold in. YDL178wp was purified as described in the materials and methods. After the gel filtration, SDS-PAGE and Western blotting were performed and examined its molecular weight of them.

Figure 2 Oligomerization of the L-form. (A) Mitochondrial fraction-derived sample (300 mg) and (B) post-mitochondrial fraction-derived sample (50 mg) were subjected to the gel filtration chromatography Superdex 200. Peak fractions were collected and analyzed by Western blotting using anti-histidine antibody. Allows exhibited fraction 19 (S-form) and fraction 4 (L-form), respectively.
Next, we examined the protein-unfolding activity of eluted fractions, in the presence of ATP, using pro-alpha factor (pαF) as a substrate for a protein-conformation modifying assay with trypsin susceptibility. Low-concentrate trypsin (200 ng/ml) did not degrade paF, it was protected and visualized as a band (Figure 3A & B) (lanes 2) as much as the control (Figure 3A & B) (lanes 1). When we added the purified samples from the mitochondrial monomeric fraction (Figure 2A) (fraction 19), it did not change the susceptibility of trypsin, the substrate was also protected (Figure 3A) (lane 3) indicating that the monomeric protein did not have the activity. On the other hand, the addition of the post-mitochondrial fraction (Figure 2B) (Fraction 4), the structure of pαF was unfolded and thus the trypsin susceptibility was increased (Figure 3B) (lane 3) suggesting that the oligomeric form contained the activity as we described before.10 To investigate the activity using a more highly aggregated substrate, we used ScN2a cells,13 which chronically infected the abnormal form of prion strain 22L.14,15 When the ScN2a cells were treated with proteinase K in the presence of detergents, detergent-insoluble aggregated fragments were detected that corresponded to 0, 1 and 2-sugar chain modifications, respectively (Figure 3C) (lane 1). Pre-incubation with samples from the post-mitochondrial membrane fraction under the presence of 1 mM of ATP resulted in the disappearance of the aggregated bands (Figure 3C) (lane 3), while samples from the mitochondrial fraction did not have the activity (Figure 3C) (lane 2), three bands of prion protein were resistant to proteinase K as well as the control (Figure 3C) (lane 1). Without proteinase K, each lane of panel C contained the same amount of the proteins.
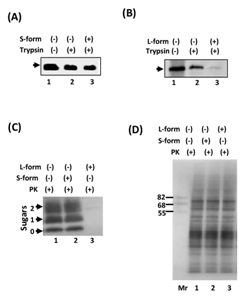
Figure 3 Existence of protein-unfolding activity in the L-form. (A) Lane 1 shows the amount of pαF used in this experiment. Low concentrates trypsin (200 mg/ml)-protected band was visualized (without S-form, lane 2). 500 ng of S-form was added to the assay mixture then the substrate was visualized as the protected band from the digestion as much as the control (lane 3). (B) Lane 1 shows the amount of pαF used in this experiment. Treatment with the L-form (500 ng) increased the trypsin susceptibility of the substrate (lane 3) compare to the control (without L-form, lane 2). (C) Proteinase K treatment of ScN2a cells with S- or L-form. Preincubation with S-form did not change the protein-digested pattern (lane 2) compared to the control (lane 1). L-form treatment strongly decreased the band, the sensitivity of proteinase K was increased (lane 3). (D) Without proteinase K, each lane of panel C contained the same amount of the proteins. 10 mg of proteins was added to each lane.
Finally, to examine the molecular weight of purified samples, we performed Western blotting using anti-histidine antibody. As shown in Figure 4A, mitochondrial derived purified samples exhibited 55-KDa of the smaller sized band as S-form (Figure 4A) (lane 1). Meanwhile, post-mitochondria derived purified sample was detected as 59-KDa of L-form, 55 KDa S-form was observed only slightly in the same fraction (Figure 4A) (lane 2). Since the hexahistidine-tag was added to the C-terminals of the protein and the bands were detected with the anti-histidine antibody, this molecular sift seems to be caused by the deletion of the N-terminal region. Therefore, next we have analyzed the N-terminal amino acid sequence of the S-form using the Edman degradation method16 and found that the S-form was processed between the N-terminal amino acids Y36 and S37 (Figure 4B). Processing of this position is completely identical to the cleavage site of presequence of YDL178wp, which can be recognized by the mitochondrial processing protease Icp55.17 In accordance with this, molecular size of the L-form was same as the rabbit reticulocyte lysates synthesized precursor protein (Figure 4A) (lane 3). These results clearly showed that the S-form was the mitochondrial mature protein. The protein coded to YDL178w in S. cerevisiae was first identified as an actin-interacting protein 2 using yeast two-hybrid system6 Later, it was re-identified as a mitochondrial protein D-lactate dehydrogenase 2;7 however, physiological function of this protein still not has elucidated. Meantime, in the course of searching protein-unfolds from yeast cells using an in vitro protein conformation-modifying assay system with the substrate of pro-a factor, we serendipitously identified the activity1,2,5 It was also YDL178w gene product, making a C-terminal residue-dependent oligomeric form consisting of 10-12 monomeric proteins and localized yeast bud necks in the log phase.10
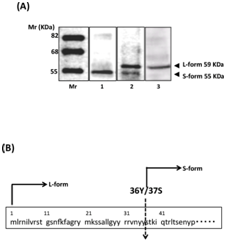
Figure 4 Existence of unprocessed form of the gene product of YDL178w. (A) The mitochondrial fraction-derived (lane 1) and post-mitochondrial fraction-derived (lane 2) samples underwent SDS-PAGE and Western blotting using anti histidine antibody. Rabbit reticulocyte lysates synthesized YDL178wp exhibited as the same size of the L-form (lane 3). 7 mg of proteins was added in each lane. Dr. Western (Oriental Yeast) was used as a molecular weight marker. (B) Amino acid sequence around the N-terminal of YDL178wp.The S-form was exhibited mature sized protein, which possessed mitochondrial presequence.
In this article we have examined two types of YDL178wp molecules, and found that the mitochondria-derived molecule is cleaved at the site of 36Y/37S, which is identical to mitochondrial processing protease cleavage site in the mitochondrial matrix.17 S-form protein did not make an oligomeric form nor did it exhibit protein-unfolding activity. On the contrary, the post-mitochondrial membrane fraction-derived protein exhibited full length of protein, indicating that it possessed the presequence region. Oligomeric form of the protein; Unfoldin also modified even the highly aggregated prion protein structure; therefore, the sensitivity of proteinase K against prion protein significantly increased. Thus, we concluded that the monomeric protein is localized mitochondrial matrix and functions as a Dld2p, while; the oligomeric protein is localized at the outside of organelle and functions as Unfoldin with the robust protein-unfolding activity. Physiological function of Unfoldin has not clearly known, however, its characteristic relation between the localization and protein-unfolding activity5,10 would be the clue as to solve this problem.
Atypical intracellular presentation of proteins with various functions, such as our case, is so called protein moonlighting or gene sharing.8,9 This was thought that one of the economical and existential strategy for genes to get the ability to perform multiple functions with minimal genes during the course of evolution. On this point, as Butler et al pointed out,18 finding of the number of moonlighting proteins would be more increase in the future with the more development of experimental techniques such as proteomics experiments.19-21 In fact, a lot of proteins possessing the characterization of protein moonlighting have been identified so far; e.g. glucose-6-phosphate isomerase as neuroleukin,22-25 the cytokine secreted from T cells as metaroprotease 1,26 oncogene nm23 as nucleoside-triphosphate (NDP) kinase27 lens crystalline as lactate dehydrogenase (LDH).28 Furthermore, recently, a lot of alternative transport/secretion pathways have found out as the unconventional pathway,29-31 and thus the dogma of protein transport have been changing. In this respect, the phenomenon such as protein moonlighting32-34 would be more noted in the near future, however, it would not be surprising if some enzyme acquired additional useful functions at the unusual localization because it seems an ingenious strategy of genes to survive during the evolution.
We have found that yeast gene product YDL178wp has two types of molecules; the mitochondrial mature sized protein as S-form and the membrane localized full-length form as L-form. Oligomeric form of YDL178wp exhibited protein-unfolding activity consisted of L-form of proteins and designated as Unfoldin; meanwhile S-form protein was processed at the N-terminal end by mitochondrial processing protease, Icp55. This atypical localization and activity would be represented protein moonlig.
None.
The author declares no conflict of interest.

©2017 Monkawa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.