MOJ
eISSN: 2374-6912


Recent studies have shown that urinary micro-vesicles (MVs) constitute a novel cellular source of biomarkers for kidney diseases. Although the available ultracentrifugation and ultrafiltration protocols effectively isolate urinary MVs, these procedures are ineffective for MVs isolation from urine containing abundant soluble proteins as found in different kidney diseases. Hence, we present a simple and convenient gel sieving chromatography based method that improves the isolation of MVs from urine containing abundant soluble proteins. Spot urine was collected from type-1 diabetes mellitus patients (albumin to creatinine ratio, ACR>300mg/g, proteins=4+) and non-proteinuria healthy subjects (ACR<30mg/g, proteins=nil). MVs were concentrated using ultracentrifugation followed by gel exclusion column chromatography. Urinary MVs fractions were collected and checked on Western blot using exosome-specific antibodies. The results showed significant removal of soluble proteins from MVs of proteinuria. Hence, this technique represents an easy and cost effective approach that could be efficiently utilized for partial purification of MVs from urine containing high proteins.
Keywords: exosomes, wilms’ stumor, tsg101
MVs, micro-vesicles; THP, tomm-horsfall protein; DTT, dithiothreitol
Micro-vesicles (exosomes and microparticles) are released by renal tubules in the urine and constitute rich source of renal biomarkers for example: Wilm’s tumor-1 (WT)-1, podocalyxin, α1-antitrypsin, aminopeptidase N, vasorin precursor, ceruloplasmin.1,2 Exosomes (30-100nm) are formed by merocrine synthesis (inward budding and exocytosis) while microparticles (100-1000nm) are formed by apocrine synthesis (surface shedding). Normal urine micro-vesicles (MVs) can be concentrated by ultracentrifugation and ultrafiltration3,4 but these procedures are ineffective in case of high proteinuricurine (protein >4+) as large number of other soluble proteins are retained with MVs.5 These abundant proteins mask the markers of interest.5 We examined two MVs preparations, one from type 1 diabetic patient’s urine containing highly abundant soluble proteins and one from normal urine. Characteristics of the urine from the diabetic patient consisted of; specific gravity=1.015, pH=7.5, Proteins=4+, Erythrocyte=2+, ACR>300mg/g. While normal urine characteristics were; specific gravity=1.005, pH=7, Proteins=nil, Erythrocytes=nil, ACR=8. MVs where prepared as previously described using the standard ultracentrifugation methods.3 Type-1 diabetic MVs and normal urine MVs preparations were examined via SDS-PAGE and immuno-recognized with MVs specific anti-tumor susceptibility gene 101 (TSG101)2,6 antibody using Western blot. A large background of unwanted soluble proteins was observed on Western blot with macro-proteinuria MVs as compared to MVs isolated from normal urine (Figure 1). These abundant unwanted soluble proteins retain with MVs proteins and create problems in biomarker search during kidney diseases leading to high proteinuria urine. In order to purify MVs from high soluble proteins, we used gel sieving column chromatography method.

We concentrated MVs using differential centrifugation as described previously.2,7 Briefly, 200ml urine sample was centrifuged at 1,500xg at 25°C followed by 17,000xg for 10min at 37°C. The 17,000xg supernatant was saved (supernatant ‘A’) and 17,000xg pellet was re-suspended in isolation solution (250mM sucrose, 10mM triethanolamine (pH 7.6) containing dithiothreitol (DTT) (200mg/ml) at 37°C for 5-10min. During incubation, sample was thoroughly mixed using a vortex at an interval of every 2-3min till the solution became transparent (approximate time~15min). Isolation solution was added to the sample to a final volume of 8ml and centrifuged again at 17,000xg for 15min at 25°C. The supernatant obtained (supernatant ‘B’) was added to supernatant ‘A’ and named supernatant ‘C’. Supernatant ‘C’ was finally transferred to ultra-centrifuge tubes (Beckman coulter, PA #344367, USA) and run in an ultracentrifuge (Beckman coulter LE80, USA) at 200,000×g for 1hour at 25°C to concentrate MVs pellet. Earlier studies show that Tomm-Horsfall Protein (THP) forms a meshwork around MVs and sediment along with MVs at 17,000xg centrifugation step.7 THP, also known as uromodulin, is a glycoprotein produced by thick ascending limb of the loop of Henle and abundantly secreted to the urine. DTT treatment to 17,000xg pellet released MVs entrapped into THP meshwork. The released MVs were then sediment following ultracentrifugation. After ultracentrifugation, the supernatant was discarded and MV pellet was suspended in 500µl Tris-HCl, pH 7.4.
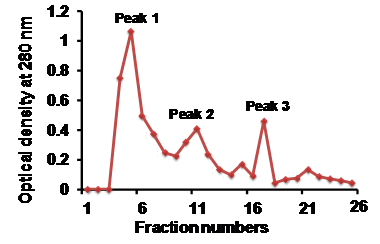
Sephadex G-25 beads were weighed and soaked overnight in 20mM Tris-Cl, pH 7.4. A clean glass column was clamped, packed with soaked sephadex G-25 beads and equilibrated with 20mM Tris-HCl, pH 7.4. The bed volume of the column was calculated as 8.0ml. The MVs pellet, suspended in Tris-HCl, pH 7.4 was applied onto the column and allowed to run with Tris-HCl (20mM, pH 7.4) buffer at a flow rate of 1min 20sec per fraction (1.0ml per fraction). Optical density at 280nm of all fractions were measured, (Figure 2) depicts the line chromatogram of all collected fractions. Three peaks were observed and identified as; peak 1, peak 2, and peak 3. All the fractions were lyophilized and concentrated in suspension solution (1.5% SDS and 50mM Tris-HCl; pH 6.8). Laemmeli sample buffer (2x) was added to concentrated fractions and thoroughly mixed using a vortex. The fractions were then incubated at 60°C for 25min and some of the fractions (5, 7, 9, 11, 15 and 21) were processed for Western blot using TSG101 antibody (Abcam, USA). Western blot analysis showed specific TSG101 band with fractions 11th, 15th and 21st and contamination was observed infractions 5, 7 and 9. This result represented that after fraction 11 the preparations contain pure urinary vesicles (Figure 3).
Pooling active fractions; pool I (5-10), and pool II (11-21) allowed us to compare the purification process and determine if other biomarkers could be detected and evaluated. The pooled fractions were analyzed and compared via SDS-PAGE and Western blot processing using apoptosis-linked gene-2 interacting protein [Alix, Abcam USA(another microvesicles marker)] and WT-1[Abcam, USA (proteinuria marker; 2)]antibody. Western blot analysis represented that purified pool II showed the presence of Alix and WT-1 bands. Purified pool I could not produce specific bands of Alix and WT-1 that showed the contamination of soluble proteins in pool I (Figure 4). We have earlier reported the presence of WT-1 in urinary exosomes as nephropathy marker in type -1 diabetic population.2 In earlier studies, Rood et al.5 used HPLC silica column for the purification of nephrotic urine MVs and obtained MVs-related proteins in nephrotic urine. However, our method is cost effective, does not require sophisticated instrumentation facility and easy to perform. It could be used in routine use for effective isolation of MVs from abundant protein containing urine (protein 4+) and thus useful for biomarker identification.
This work was supported by SGPGI- Intramural research grant to ST and Indian Council of Medical Research associate fellowship to AK (3/1/3/5/2011-RHN).
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.