MOJ
eISSN: 2641-9297


Research Article Volume 1 Issue 2
Department of Agricultural and Food Engineering, University of IIT Kharagpur, India
Correspondence: Goswami TK, Department of Agricultural and Food Engineering, University of IIT, Kharagpur, India
Received: October 18, 2017 | Published: March 29, 2018
Citation: Raj V, Goswami TK. Use of phase change material (PCM) for the improvement of thermal performance of cold storage. MOJ Curr Res & Rev. 2018;1(2):49-61. DOI: 10.15406/mojcrr.2018.01.00010
The project deals with the development of cold storage facility for improvement of its thermal performance by application of polyethylene glycol 400 (PEG400) as a Phase change material (PCM). PCM are the materials which generally have low melting point and they change their phase by absorbing the latent heat from the system so as to maintain the temperature of system. Present study was done to determine the rise in the temperature of air inside the cold space in the situations like frequent door openings and electrical power failures. The main aim of this project was to observe the lowering in the rise of temperature when there is PCM used inside the cold space of the cold storage. The experimental result showed that with the application of PCM temperature maintained inside the cold space was 1-4 °C lower than without PCM in case of frequent door openings. And also in case of electrical power failures with the use of PCM, temperature maintained was 1-4.5 °C lower than without application of PCM. The maximum power savings observed in case of door openings was 1021.88 kJ/h when the door was opened 3 times for 10 seconds with an interval of 20 minutes each and for electrical power failure for 1 hour the power saved was 3.115 kJ/h which was higher than the rate of energy absorbed by PCM in other cases.
Keywords: Cold storage, Phase change material (PCM), Polyethylene glycol 400 (PEG 400), Door openings, Electrical power failures
Background
Latent heat storage using phase change materials (PCMs) is one of the most efficient methods to store thermal energy. Therefore, PCM have been applied to increase thermal energy storage capacity of different systems. The use of PCM provides higher heat storage capacity and more isothermal behaviour during charging and discharging compared to sensible heat storage. Moreover, thermal energy storage (TES) systems for both heat and cold are necessary for good performance of many industrial processes. High energy storage density and high power capacity for charging and discharging are desirable properties of any storage system. These storage systems have been studied for many years addressing different problems of the used materials such as low thermal conductivity and segregation of the PCM. As regards to the criteria for selecting a suitable storage medium, the phase change materials (PCMs) are very attractive candidates in a thermal energy storage system and has drawn huge interest in recent years.1 The criteria include but not limited to the thermo-physical benefits, environmental effects and economy. The use of PCMs as storage media is by far the most effective and efficient way to storing thermal energy by exploiting the enthalpy of phase change as the storage mechanism.2 Among all possible phase change processes, the solid-liquid phase change is considered as the most suitable form for TES applications. The use of PCM in TES has many advantages over other forms of energy storage mediums, especially the sensible heat storage.3 Because of PCM’s high energy density (amount of heat energy storage per unit mass), it is possible to have much smaller and compact storage facilities compared to sensible heat storage types. The characteristic ability of PCM’s narrow temperature range during thermal energy charging and discharging periods has made it a suitable candidate for applications where smaller range of temperature variations are important.
Thermal energy storage (TES)
Heat and thermal energy are often used as synonyms but based on strict thermodynamic definition, they are not the same. Thermal energy describes the thermal content of a substance or a system, while, heat is the content of thermal energy that is transferred from one material or system to another as a consequence of the temperature difference between the two. Thermal energy can also be generated, at the expense of converting energy from one form to another. In general, temperature is an indicator which determines the ability for a physical system (i.e. certain amount of mass of a substance) to transmit thermal energy to another physical system. Presence of heat can only be realized when there is a difference in temperature and the energy crosses the system boundary. Thus, the heat transfer deals with the determination of the rates of thermal energy transfer by taking into account the variations in temperatures. TES system is in practice for centuries. It is essentially a means by which thermal energy is ‘hold’ for a certain period of time. Storing thermal energy (in the form of heat or cold) in an appropriate form and in a suitable media when it is surplus, and extracting the same at a later time when needed is the basic principle of a TES. TES is proved to be an effective and practical solution to address the issues of intermittency and can play a vital role in efficient energy management system. The main purpose of TES is to overcome the mismatch between the energy generation and its utilization.4 TES is gaining enormous importance and is becoming a noteworthy topic due to its growing importance in various areas of engineering and general applications. TES has the enormous potential in conserving energy which in turn can minimize the carbon footprint on the environment.
Benefits of thermal energy storage (TES)
The primary benefits of a TES are summarized below:
Physical methods of thermal energy/heat storage
There are three basic and distinct modes of heat transfer used for storing thermal energy; the sensible heat, the latent heat or the thermo chemical energy.5 The sensible component accounts for the translational, rotational and vibration motions of the atoms and molecules. The latent component is the consequences of the intermolecular forces of the material that influence the phase change between solid, liquid and gaseous states. The chemical component accounts for the energy stored in the chemical bonds between atoms.6
Sensible heat storage
When the energy is stored or released by increasing or decreasing the temperature of the storage material respectively, it is considered as the sensible heat storage. The amount of thermal energy stored is dependent on the temperature change in the material, mass of the material, m (kg) and its heat storage capacity Cp (kJ / kg °C) and can be expressed as:
(1.1)
Where, T1 and T2 are the lower (initial) and upper (final) temperature levels between which the storage system is operating. Benato et al.7 however has showed improved method to consider sensible heat.
Latent heat storage
Latent heat storage system involves material’s phase transition i.e. the energy is stored or released due to the change in the enthalpy of the phase change. Due to the involvement of the phase change, the thermal energy storage operates almost isothermally at the phase change temperature of the material. The amount of thermal energy stored, Q in this case depends upon the mass, m and the latent heat of the phase change of the material and is expressed in the form:
(1.2)
where, m is the mas of storage material (kg), and Δhm is the phase change enthalpy or alternately, the latent heat of fusion/solidification (kJ/kg).
Total amount of heat storage:
When a material is heated from its solid state to complete melting and beyond (over a range of temperatures Ts to Tl that passes through the melting point Tm), the total thermal energy stored in the form of heat can be calculated as:
(1.3)
where, Cps and Cpl represents the specific heats of the solid and liquid phases and Ts, Tl and Tm are the temperature of the solid, liquid and melting/fusion of the material respectively. During the initial stage, the PCM behave as a sensible heat storage material where its temperature increase as they absorb heat. But when the PCM reaches its melting temperature, unlike conventional sensible heat storage material, the PCM absorbs large amount of heat at nearly a constant temperature, which is in fact, the latent heat of fusion of that particular PCM. The PCM continue to absorb heat maintaining this near constant temperature until the PCM is completely melted to the liquid phase. After complete melting, the liquid PCM temperature again starts increasing above its melting temperature as sensible heat storage and continues to rise depending on the input temperature. Theoretically, all phase-change processes can be employed for storage and release of energy. There are four types of changes between phases that are of significance: solid-liquid (melting and solidification), liquid-gas (evaporation and condensation), solid-gas (sublimation and re-sublimation), and solid-solid transformations. The absolute value of latent heat of phase change for a solid-solid transition is usually less than that of solid-liquid conversation. Solid-gas and liquid-gas transitions are generally not employed for energy storage, in spite of their high latent heat, only because of their (gases) occupations of large volumes, requiring a big space for storage or conversely stored at high pressure to keep smaller volume, which introduces critical and costly constraints on the storage design. Hence, a solid-liquid transition, which involves only a small change in volume and negligible pressure issues, is quite promising for phase change energy storage.
Comparison: Sensible and latent heat TES
Each system has its own advantage and disadvantage, which may vary drastically with changes in the thermos-physical conditions as well as the adaptability.8 Thus; it is difficult to generalize the superiority of one over the other. Several explicit factors in a given application dictate the suitability and selection of a heat storage type. These factors may include type, size, location, duration, frequency, repeatability etc. of the TES’s use. In other words, choice of TES is exclusively need specific. Although the heat transport and the design philosophy of a latent heat thermal storage system (LHTES) are much more complex than sensible heat thermal storage system (SHTES), from the technical point of view, LHTES has some major advantages over sensible type. Firstly, LHTES has the advantage of storing large amount of heat (energy storage density) in the same amount of mass of the thermal storage media by exploiting the enthalpy of phase change. Secondly, an attractive feature of a LHTES system is that, the operating temperature can be anchored in a narrow band around the phase-change temperature of the media, making the system very suitable for most of the applications. Another notable advantage the LHTES possess is that, even if there is a small temperature difference (ΔT) between the sink and the source, a large amount of heat can still be possible to store. Whereas, the effectiveness of a SHTES system solely depends on the temperature differences, i.e. higher the ΔT, more is the heat storage as seen in Figure 1. SHTES has the advantage of being relatively simple in design and cheap, but the energy storage density is low and there is a gliding discharging temperature that immensely affect its performance. LHTES is found in a wide range of applications, which include space heating and cooling, metal processing, thermal energy storage, temperature control in miniature electronics devices, food processing and storage, spacecraft thermal system applications etc.
Requirements of a LHTES
A single PCM or a design of a LHTES cannot fulfil all the desired requisites. During designing a LHTES system, various requirements need to be addressed. These include: high energy density of the storage material (storage capacity), good heat transfers between the HTF and the storage material, mechanical and chemical stability of the storage material, compatibility between storage media and the container material, complete reversibility and the number of cycles, low thermal losses during the storage period, impact on environment and easy control. Moreover, cost, operational strategy, maximum load and integration with other systems are also important.
Complexities with LHTES
As mentioned earlier, the LHTES has some excellent favourable criteria, but they have complex heat transfer aspects and have difficulty in tracking and predicting the solid-liquid interface during the phase change process making it a really challenging problem. These complexities and challenges can be summarized as:
A good understanding on these aspects is of immense importance for LHTES system design perspectives. However, there is scarcity of available information in the literature concerning these issues.
Concerns with the thermal conductivity of PCM
Almost all phase change materials (PCMs), whether organic or inorganic, have a drawback of having low heat transfer rates during melting and freezing processes due to their inherent low thermal conductivity. Low thermal conductivity in PCM hinders the heat transfer process within its domain by prolonging the charging (heat addition) or discharging (heat rejection) period. This problem not only drastically affects the melting and solidification performance of the LHTES system, but also limits their widespread use as latent heat storage material. The thermal conductivities of organic and inorganic materials usually swing around approximately 0.2 W/m°C and 0.5 W/m°C, respectively. To have these PCMs successfully utilized commercially, some mechanism needs to be incorporated that can enhance the thermal conductivity. Enhancing the heat transfer rate through a PCM is a present day challenge. Different approaches have been proposed to enhance the thermal conductivity of PCM. For example, the placement of metal structure (i.e. fins) inside a PCM, use of porous material, metal foams, carbon fibres and dispersion of high thermal conductivity nanoparticles in the PCM are among the common techniques used to enhance the effective thermal conductivity of PCMs. Although there are many merits in these methods/applications, however, most of these enhancement techniques suffer from increased weight and volume in the system, except for the use of nanoparticles. Nanoparticles possess physical and chemical properties, which are quite different from their bulk form.
Producing various types of nanoparticles has become cheaper due to rapid advancement in nanotechnology. There is a growing use of nanotechnology in different engineering, electronics and industrial processes. The presence of a mere amount of nanoparticles in the PCM significantly increases the effective thermal conductivity of the PCM, and consequently enhances the heat transfer characteristics. A fluid containing Nano particles, generally less than 100 nm sizes is termed as Nano fluid. Nano fluids are prepared by dispersing such tiny particles (1-100 nm) in a base fluid such as water, ethylene glycol, propylene glycol, oil and other conventional heat transfer fluids. Preserving the intended properties, holding nanoparticles in the fluid suspended after repetitive cycles and the cost of bulk volume of nanoparticles are the few major problems of widespread use of these nanoparticles. Based on the above facts the following objectives have been targeted for the present study:
Classification of PCM
The phase change materials (PCMs) are generally divided into two groups: organic and inorganic. Inorganic compounds show a volumetric latent TES capacity twice that of organic compounds. But the organic substances could serve as important heat storage media because of several advantages from their natural properties like, ability to melt congruently, self-nucleation, non-toxic and non-corrosiveness, environment friendly etc. Alkanes and paraffin are in the latter category. Figure 2 shows various common materials for sensible and latent heat storage systems.
Paraffin
Paraffin is one of the most studied phase change materials in thermal energy storage application because of their availability, relatively low-cost and non-corrosive nature. Paraffin is normally composed of straight long chain alkanes, the heat release and melting point of this paraffin depends on the chain length. Though it has few advantages but the bottlenecks i.e. low thermal conductivity and moderate flammability made it only applicable to small scale and only for small temperature application.
Non-Paraffin
Among the non-paraffins some of the fatty acids, alcohol and glycol are suitable for thermal energy storage, but all of the non-paraffin has a major drawback, that is their flammability and they should be handled carefully under high temperature environment. Some of the potential organic PCM are: Ammediol (melting point 112 °C and heat of fusion 285 kJ/kg), neopentyl glycol, isomalt, adipic acid, tromethamine etc.
Inorganic Salt and Salt Composites
Salt hydrates are the most extensively studied PCM because of their highest potential in terms of moderately higher thermal conductivity, inexpensiveness and safe operation. But one of the major challenges of using salt hydrates PCM in TES is its incongruent melting. When salts are not soluble by its associated water then incongruent melting occurs leaving the salt solution supersaturated after melting. This phenomenon creates loss of salt due to settling after several melting-freezing cycles. But there are ways to avoid it such as using stirrer, using water so those melt portions do not get supersaturated. Liu et al.12 in his study listed several inorganic salt composites as a potential PCM storage application due to their higher energy density, heat of fusion and relatively lower cost. Among those NaNO3, NaOH, KNO3, KOH, Na2CO3 have been used extensively for research purpose. In terms of cost KOH proved to be the best choice and in terms of energy density NaNO3 proved to be the best choice. NaNO3 proved to be the best choice for producing high pressure steam around 100 bar. Sole et al.10 reported that though fluoride salts have much higher heat capacity but in terms of cost and material compatibility, they are not the best choice for PCM salt selection and same is the case for KOH which is very cost effective but impose very high corrosion with the containing material.
Metal and Metal Alloys (Metallics)
Metals and metal alloys are attracting researcher’s attention now days because of their some highly favourable characteristics over salt hydrates such as high thermal conductivity, higher heat of fusion per unit of volume and small volume change during phase change. But in most of the case they are not in the final consideration list because of their weight penalties as well as cost. But recently as a thermal conductivity enhancement technique these metals are used with the other PCM materials as additives. Among the metals and metal alloys AL-Si alloy (88%Al, 12%Si) and 6Al-Mg-Zn (60-34-6) alloy has been tested the most because of their higher energy density and thermal conductivity.
Donhowe & Hartel11 studied ice recrystallization during bulk storage of ice cream in containers. The temperature of the freezer was controlled with and without fluctuations (with different levels of temperature control of ± 1.0 °C and ± 0.01 °C) at set temperatures between -15 and -5 °C. They concluded that recrystallization rate increased with the increase in storage temperature and extent of temperature fluctuations.
Phimolsiripol et al.12 also studied the effects of freezing and temperature fluctuations on frozen dough and bread quality. The rates of quality and weight losses were significantly greater when temperature fluctuation was high (-18 ± 5 °C). Since temperature fluctuation is unavoidable, the same authors suggested that temperature variations should be kept minimum and not higher than ± 3 °C.
Gormley et al.13 studied the effect of temperature fluctuation (cycling between -30 and -10 °C) versus constant frozen storage temperatures regimes (-60 or -30 °C) on quality parameters of selected food products. Temperature fluctuation is expected to produce stress damage and other deleterious effects such as fat oxidation and changes in colour and texture. Moreover, temperature fluctuation could lead to high peroxide and free fatty acid contents in food.
Nagpo et al.14 studied the effect of freezing and thawing rate on moisture drip loss from samples of pork. They compared a fresh sample to a sample frozen at slow and fast freezing rates and the drip loss was significantly reduced in the sample frozen at high rate.
Onyejekwe15 incorporated PCM into a domestic freezer. Their work was based on using an easily available and very cheap PCM (NaCl + H2O) for thermal energy storage (TES). However, such type of PCM suffers severe corrosion and sub-cooling.
Wang et al.16,17 developed and tested a prototype refrigeration system that incorporates PCM. They placed a PCM heat exchanger at different locations in the refrigerator such as at the back the compressor, and the condenser. The experimental results showed that the integration of PCM heat exchangers into the refrigeration system could improve the COP of the system by 6-8%.
Wang et al.18 developed a dynamic novel system which can be used to design and optimize the performance of the refrigeration system.
Farid et al.19 designed a method of a novel dual evaporator for domestic refrigerator with PCM which provided thermal storage in order to improve food quality and prolong compressor off time.
Azzouz et al.20 Using numerical investigations, showed that the addition of a thick slab of PCM on the back side of a refrigerator evaporator may result in a higher evaporating temperature leading to 5-15% increase in the COP, a significant decrease in the number of starts and stops of the compressor, and lower freezer indoor air temperature.
Azzouz et al.21 experimentally showed that the use of PCM behind the evaporator in a household refrigerator improves its performance and allows several hours of refrigeration without the need for electrical power supply.
Cheralathan et al. 22 Using bigger chambers, carried out an experimental investigation on the performance of an industrial refrigeration system integrated with encapsulated PCM based on cold TES system. In the experimental set-up a vertical storage tank is integrated with the evaporator of the vapour compression refrigeration system. They reported the effects of inlet HTF temperature on system performance and demonstrated the useful addition of PCM TES to the system.
Gin et al.23 investigated the incorporation of PCM panels placed against the internal walls of a domestic freezer to maintain stable temperatures in the presence of heat loads. Moreover, energy consumption tests showed that the inclusion of PCM into the freezer decreased the energy consumption during a defrost cycle by 8%, and by 7% during door openings. Furthermore they observed that the introduction of PCM improved the quality of the frozen foods during storage.
Based on the above facts the following objectives have been targeted for the present study:
Model cold storage
For the application of PCM to improve thermal stability during power loss in cold store, a model cold cabin having storage space dimensions 0.61 m × 0.60 m × 0.83 m and a storage volume of 303.78 litre was used. The boundary condition for load calculations was taken as 45 °C outside ambient temperature and 70% RH. The temperature and relative humidity that should be maintained inside the storage space was 4 °C and 95% respectively. Thickness of wall was 9 cm containing 3.5 cm thick sheet of stainless steel, 5 cm thick insulation of PUF and 0.5 cm mild steel plate. The door was 6 cm thick containing 0.5 cm thick stainless steel sheet, 5 cm thick PUF insulation and 0.5 cm thick mild steel plate. In-between door and storage space there was a rubber seal of thickness 1.5 cm which helped to provide leakage.
Heat load calculations
The sources of heat coming inside the cold space of our model cold cabin was due to transmission through walls, respiration heat of the product and infiltration heat due to door openings. These heats on summation gave the total heat of the evaporator.22 Some design parameters which were considered for calculation are appended below:23
Heat transmission through walls:
The heat transmission through walls can be calculated by the following formula:
𝑄 = 𝑈𝐴 (𝑇𝑜−𝑇𝑖 )
(2.1)
where, U = Overall heat transfer coefficient, W/m2 °C
A = Area, m2
(𝑇𝑜−𝑇𝑖) = Temperature gradient, °C
ho = Outside air convective heat transfer coefficient, W/m2 °C
hi = Inside air convective heat transfer coefficient, W/m2 °C
xi = Thickness of the materials, m
ki = Thermal conductivities of the materials, W/m °C
For outside air
, Nre < 30000
W/m2 °C
For inside air
, > 3000
W/m2 °C
For walls,
U = 0.427 W/m2 °C
Area of wall = 2 (0.74 × 0.91 + 0.61 × 0.74) + 0.91 × 0.61 m2
= 2.8047 m2
Heat load from walls:
Q1 = 0.427 × 2.8047 × (45-4)
= 49.1 Watts @ 50 W
For door,
U = 0.428 W/m2 °C
Area of walls = 0.79 × 0.91 m2
= 0.7189 m2
Heat load from walls:
Q2 = 0.428 × 0.7189 (45-4)
= 12.61Watts @ 13 W
Heat load transmitted = Qt = Q1 + Q2 = 63 W
Also, specific heat in potato at the time of entrance Cp = 3430 J/kg/°C (assuming entrance temperature to be 25 °C)
Come up time = 3 hours = 10800 seconds
So, heat transferred at initial state = (m × Cp × ∆T) / time
Qinitial = 162 × 3430 (45-4) / 10800
= 2109.45 W
Heat Load through respiration
Storing 162 kg potatoes having heat of respiration to be 0.1 kJ/kg/h would generate heat as given below:
Qr = m × respiration heat
Qr = 162 × 0.1×1000/3600
Qr = 4.523 W
Heat load due to infiltration
Considering the rate of air change was 4 times fresh air change per day
Outside air enthalpy, hout = 99.173 kJ/kg (at 45 °C DBT & 30 °C WBT)
Inside air enthalpy, hin = 21.79 kJ/kg (at 4 °C and 95% RH)
Enthalpy difference = (99.173 – 21.79) kJ/kg
∆h = 77.383 kJ/kg
= (0.254 × 4) / (24 × 3.6)
= 0.01176 l/s
Standard formula for heat load due to infiltration = 1.2 × l/s× ∆h W
Qinf = 1.2 × 0.01176 × 77.383 W
Qinf = 1.092 W
Total heat load
Total heat load are the summation of transmission, initial, respiration and infiltration heat loads calculated above:
Total heat load = Qt + Qinitial + Qr + Qinf
Qe = 2176.775 W
To overcome experimental errors, we took factor of safety of 10%
Thus total heat load inside the evaporator was (2176.775 + 217.67) = 2394.445 W @ 2500 W = 2.5 kW
Calculation for COP
Evaporator temperature, Te = 4 °C
Pressure correspond to Evaporator temperature, Pe = 3.152 bar
Saturated vapour enthalpy corresponding to Evaporator temperature, h1 = 401.1 kJ/kg
Saturated vapour entropy correspond to Evaporator temperature, s1= 1.7257 kJ/kg = s2
Condenser temperature, T2 = 60 °C (superheated)
Pressure correspond to h3, P3 = 5.07 kPa = P2
Saturation Temperature corresponds to P3 = Tc = 18 °C = condenser temperature
Enthalpy at Tc = hc = 408.9 kJ/kg (vapour)
Specific heat of R134a = 0.204 kCal/kg °C = 0.853 kJ/kg °C
Enthalpy at point 4; h4 = hf (liquid) + x hfg
(Assuming x = 0.1, because refrigerant entered in evaporator was almost in liquid state)
h4 = 205.4 + 0.1 × 195.7
h4 = 224.97 kJ/ kg = h3
h2 = hf (vapour) + cp ∆t
h2 = 408.9+ 0.853 × (60-18)
h2 = 444.73 kJ/kg
For mass flow rate,
Qe = mr (h1 - h4)
2.5 kW = mr (401.1 – 224.97)
mr = (176.`13)/2.5
mr = 0.0142 kg/s
At 4 ⁰C density of R134a vapour, ρ = 16.569 kg/m3
vr = mr / ρ
vr = 0.857 l/s
Work done by the compressor,
Wc = mr (h2 – h1)
Wc = 0.0142 (444.73 – 401.1)
Wc = 619.55 W
(Assuming compressor efficiency to be 80%)
Wc = 619.55 / 0.8 = 774.43 W ≈ 775 W
Study of melting behaviour and transient heat transfer in phase change materials
The main principle of cooling with PCMs is the receiving or releasing of a high amount of cold during a phase change at an extremely low temperature difference and relatively constant temperature. This phenomenon occurs during the solidification and melting processes. During the cold storage process, the PCM is solidified and during the cold release process the PCM is melted (Figure 3). Curve A represents an actual solidification or melting process, where the slope of the curve is increased during the phase change. However, in theoretical studies the solidification or melting process appears at a constant temperature and is shown by the vertical (B) curve.
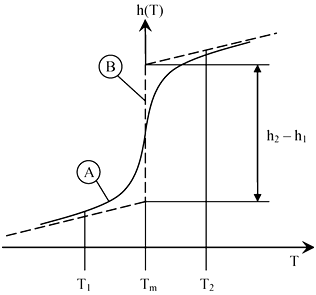
Theoretically, the total amount of cold is stored at the temperature difference is ∆T = T2 - T1 and is expressed by Equation (1)
Experimental setup
An experimental set-up for the analysis of melting temperature and latent heat transfer of different PCM were conducted.
Materials used
Material used:
Method:

Experimentation of PCM incorporation
Sets of experiments conducted with and without incorporation of PCM are shown in Tables 1 & 2 as shown below:
Results and discussions
The present study was conducted to find the effect on air temperature inside the cold space of a Model cold storage with the application of phase change material in it.
Model Cold Storage
Theoretical design is completed for a lab model cold storage containing R134a as an operating refrigerant. The design gives the data of operating parameters as follow:
Melting behaviour of PCM
The tests are run and graphs are plotted between temperature and time of two PCM namely, water and ethylene glycol gives the melting behaviour of PCM. According to theoretical concept the melting point is the constant temperature at which the phase transition takes place is not possible practically, so we are taking the average temperature of extreme temperatures on the phase transition profile.
Melting profile of water
The graph plotted between temperature and time for water for observing the melting behaviour is shown in Figure 6. From the graph we observed that the water in the form of ice initially at - 25 °C is taking heat from the water and starts melting. In the beginning, curve follows a regular trend of increasing temperature from - 25 °C up to - 4.2 °C, and then a sinusoidal behaviour is shown in the curve up to 4.2 °C which is the phase transition of ice to water. After that the curve changes trend and temperature is increasing further. To get the melting point we have to take the average of - 4.2 °C and 4.2 °C.
Melting temperature = (- 4.2 + 4.2) / 2
Tm = 0 oC
Thus melting point of ice is 0 °C (approx.)
Melting profile of ethylene glycol
The graph plotted between temperature and time for ethylene glycol for observing the melting behaviour is shown in Figure 7. From the graph we observed that the ethylene glycol in the form of solid, initially at - 22 °C, was taking heat from water and started melting. In the beginning, the curve follows a regular trend of increasing temperature from - 25 °C up to -17.3 °C, and then a sinusoidal behaviour is observed in the curve up to - 8.5 °C, which is the phase transition temperature of solid ethylene glycol to be transformed to its liquid form. After that, the curve again changes its trend and temperature increases further. To get the melting point we have to take the average of -17.3 °C and - 8.5 °C.
Melting temperature = [(- 17.3) + (- 8.5)] / 2
Tm = - 12.9 °C
Thus melting point of ethylene glycol is -12.9 °C
PCM selection
For selection of PCM, the range of temperature of air inside the storage space of cold storage is obtained from the graph between temperatures of air versus time. The PCM is then selected according to its melting temperature which should be within the range of air temperature as shown in Figure 8.
Polyethylene glycol 400 (PEG 400)
From Figure 8 & Figure 9, we find that temperature fluctuates between the temperature ranges of 3 °C to 6 °C. The PCM studied in this work was Polyethylene glycol 400 (PEG 400), which has melting temperature of 5°C as obtained from the manufacturing company Merckmillipore. The chemical formula of PEG 400 is HO(C₂H₄O)nH.
Amount of PCM required
Heat load that has to be removed from the cold space inside the evaporator = 2.5 kW
Initial heat load = 2.11 kW
Amount of heat considered to be removed, Q = 2.5 – 2.11 = 0.39 kW ≈ 400 W
Latent heat of polyethylene glycol 400, λ = 150.98 kJ/kg
Density of polyethylene glycol 400, ρ = 1125.5 kg/m3
Now, heat considered to be removed should be equal to the latent heat of polyethylene glycol 400 per hour basis.
∴ Q = m X λ
400 (J / s) = m X 150.98 X 1000
m = 400 X 3600 / (150.98 X 1000)
m = 9.537 kg
Volume (v) = mass (m) / density (r)
∴ v = 9.537 / 1125.5
v = 8.474 × 10-3 m3
∴ v = 8.5 l (per hour basis)
Design of Trays to hold PCM
The PCM containing trays were designed such that they can be placed in the shelves of the cold storage (Figure 10) (Figure 11). Their size was made slightly smaller than the cross sectional area of the shelves, leaving enough gap for air circulation inside the storage space of the cold storage. The material for the construction of trays used is GI sheet 1.25 mm thick. The total volume of each tray (40cm×40cm×2cm) was 3.2 × 10-3 and PCM in each tray was 2.8 × 10-3 m3 , occupying a total of only 2.76% of internal volume of the cold storage. Each tray weighed 3.1 kg and contained 2.833 kg of Polyethylene glycol 400; therefore the total amount of PCM inside the cold storage was 9.537 kg.
Cold storage’s inside air temperature response to door openings
A series of door openings have been done with and without incorporation of Polyethylene glycol 400 as a Phase change material. Four different tests (Table 1) were performed in order to study the behaviour of cold storage.
Test scenarios |
Number of door openings |
Time for that the door was opened for (s) |
Time between openings (min) |
Test 1a. Door opening |
3 |
10 |
20 |
Test 1b. Door Opening |
3 |
30 |
20 |
Test 1c. Door Opening |
3 |
180 |
20 |
Test 1e. Door Opening |
1 |
600 |
- |
Table 1 Set of experiments related to door openings conducted for experimentation.
Test 1a: Door opening
Test was conducted for opening the door 3 times for 10 seconds each. The duration between openings of door was 20 minutes. The graph plotted between air temperature and time is given in Figure 12: From Figure 12, it is revealed that the temperature rises on opening the door and it reaches to 17 °C when the door remained open for 10 seconds. It was observed that the use of PCM maintained the air temperature 3 °C lower (14 °C) than that without PCM.
Test 1b: Door opening
Test was conducted for opening the door 3 times for 30 seconds each. The duration between openings of door is 20 minutes. The graph plotted between air temperature and time is given in Figure 13: Figure 13 reveals that the peak of temperature rises to 21 °C during door opening for 30 seconds and it is observed that the use of PCM maintained the air temperature 1.04 °C lower than that without PCM.
Test 1c: Door opening
Test conducted for opening the door 3 times for 3 minutes each. The duration between openings of door is 20 minutes. The graph plotted between air temperature and time is given in Fig. 3.9. It is clear from Figure 14 that the use of PCM maintained the air temperature 1.11 °C lower during door openings for 3 minutes.
Test 1d: Door opening
Test conducted for opening the door for 10 minutes. The graph plotted between air temperature and time is given in Figure 15: From Figure 15, it is observed that the temperature rises to 32 °C during door opening for 10 minutes. The use of PCM maintained the air temperature 3.5 °C lower than that of without PCM.
Air temperature response to electrical power failure
A series of electrical power failures have been done with and without incorporation of Polyethylene glycol as Phase change material. Four different tests (Table 2) were performed in order to study the behaviour of cold storage.
Test scenarios |
Time of electrical power failure (min) |
Test 2a. Electrical power failure |
15 |
Test 2b. Electrical power failure |
30 |
Test 2c. Electrical power failure |
60 |
Test 2d. Electrical power failure |
120 |
Table 2 Set of experiments conducted during electrical power failure (simulated).
Test 2a: Power failure
Test conducted for electrical power failure for 15 minutes. The graph plotted between air temperature and time is given in Figure 16: From Figure 16 it is observed that electrical failure of 15 minutes could cause rise in temperature to 11.38 °C. The use of PCM maintained the air temperature 0.38 °C lower than that without PCM.
Test 2b: Power failure
Test conducted for electrical power failure for 30 minutes. The graph plotted between air temperature and time is given in Figure 17: From Figure 17 it is observed that on power failure of 30 minutes the temperature rises to 14 °C. The use of PCM maintained the air temperature 1 °C lower than that of without PCM.
Test 2c: Power failure
Test conducted for electrical power failure for 1 hour. The graph plotted between air temperature and time is given in Figure 18: From Figure 18 it is observed that electrical failure of 1 hour could cause rise in temperature to 18 °C. The use of PCM maintained the air temperature 3.1 °C lower (up to 15 °C) than that without PCM.
Test 2d: Power failure
Test conducted for electrical power failure for 2 hours. The graph plotted between air temperature and time is given in Figure 19: From Figure 19 it is observed that the temperature rises to 21 °C during power failure for 2 hours. The use of PCM maintained the air temperature 4.5 °C lower (up to 17 °C) than that without PCM.
Analysis of Power savings
For the analysis of power saving we have calculated the compressor work in both cases i.e. with and without incorporation of PCM. The net power saving due to PCM incorporation in a cold storage system can be calculated by finding the difference between the compressor work done with and without PCM. This compression work when divided by time taken for analysis gives the net power saving. The theoretical power saving can be obtained by dividing the net power saved by the efficiency of the compressor system which can be as much as 70% in this case. The detailed calculations and the results of net power saving for each set of experiments is presented below:
Power saving in case of power cut
Test 1a: Door opening for 10 seconds after 20 minutes of interval
Without PCM
Peak temperature attained, Te = 17 °C
Enthalpy of refrigerant before entering the compressor, h1 = 425 kJ/kg
Entropy of refrigerant before entering the compressor, S1 = 1.71923 kJ/kg °C
Enthalpy of refrigerant at the exit of compressor, h2 = 450 kJ/kg
Enthalpy of refrigerant at the exit of condenser, h3 = 240 kJ/kg = h4
Condenser temperature, Tc = 30 °C
Heat load in the evaporator, Qe = 2500 W
Qe = mr (h1-h4)
mr = 0.0135 kg/s
Compressor work = Wc1 = mr(h2-h1)
= 337.5 W
With PCM
Te = 14 °C
h1 = 420 kJ/kg
S1 = 1.718 kJ/kg °C
h2 = 450 kJ/kg
h3 = 240 kJ/kg = h4
Qe = 2500 W
Qe = mr (h1-h4)
mr = 0.01388 kg/s
Compressor power = Wc2 = mr(h2-h1)
= 138.88 W
Net compressor power = Wc1- Wc2 = 337.5 – 138.88 = 198.7 W
Theoretical compressor power = 198.7/0.7 = 283.86 W
Net energy saved in one hr = 0.283×3600 = 1021.88 kJ
Test 1b: Door opening for 30 s after 20 minutes of interval
Without PCM
Te = 21 °C
h1 = 432 kJ/kg
S1 = 1.7177 kJ/kg °C
h2 = 450 kJ/kg
h3 = 240 kJ/kg = h4
Tc = 30 °C
Qe = 2500 W
Qe = mr (h1-h4)
mr = 0.01316 kg/s
Compressor work = Wc1 = mr (h2-h1) = 234.4 W
With PCM
Te = 19.9 °C
h1 = 429 kJ/kg
S1 = 1.718 kJ/kg °C
h2 = 440 kJ/kg
h3 = 240 kJ/kg = h4
Qe = 2500 W = mr (h1-h4)
mr = 0.01323 kg/s
Compressor power = Wc2 = mr (h2-h1)
= 145.5 W
Net compressor power = 88.9 W
Theoretical compressor power = 127 W
Theoretical energy saved in one hr = 457.2 kJ
Test 1c: Door opening for 3 minutes after 20 minutes of interval
Similar calculations can be done
We get,
Wc1 = 264.6 W
Wc2 = 240.6 W
Net compressor power = 23.96 W
Theoretical compressor power = 34 W
Theoretical energy saved in one hr = 123 kJ
Test 1d: Door opening for 10 minutes.
Similarly we get,
Wc1 = 192.3 W
Wc2 = 197.3 W
Net compressor power = 5.1 W
Theoretical compressor power = 7.3 W
Theoretical energy saved in one hr = 26.2 kJ
Power saving in case of power cut
Test 2a: when power failure was for 15 minutes.
Heat absorbed by air per unit mass without PCM when power failure was for 15 minutes = Cp × ∆t = 1.005 × (11.38 - 5) = 6.4119 kJ/kg of air
Heat absorbed by air with PCM when power failure was for 15 minutes = 1.005 × (11 – 5) = 6.03 kJ/kg of air
Heat absorbed by PCM in 15 minutes of power failure = 0.389 kJ = 1.5276 kJ/hr
Test 2b: when power failure was for ½ hour.
Heat absorbed by air per unit mass without PCM when power failure was for ½ h = Cp ×∆t = 1.005× (14-5) = 9.045 kJ/kg of air
Heat absorbed by air with PCM when power failure was for ½ hr = 1.005 × (13 – 5) = 8.04 kJ/kg of air
Heat absorbed by PCM in ½ hr of power failure = 1.005 kJ = 2.01 kJ/hr
Test 2c: when power cut was for 1 hour.
Heat absorbed by air per unit mass without PCM when power failure was for 1 h = Cp × ∆t = 1.005 × (18 - 5) = 13.065 kJ/kg of air
Heat absorbed by air with PCM when power failure was for 1 hr = 1.005 × (14.9 – 5) = 9.95 kJ/kg of air
Heat absorbed by PCM in 1hr of power failure = 13.065 – 9.95 = 3.115 kJ/h
Test 2d: when power failure was for 2 hours.
Heat absorbed by air per unit mass without PCM when power failure was for 2 h = Cp × ∆t = 1.005 × (21 - 5) = 16.08 kJ/kg of air
Heat absorbed by air with PCM when power failure was for 2 hr = 1.005 × (16.5 – 5) = 11.56 kJ/kg of air
Heat absorbed by PCM in 2 h of power failure = 4.52 kJ = 2.26 kJ/h
Peak temperature difference was analysed with and without PCM incorporation in cold storage chamber. The benefit of introduction of PCM inside the cold storage is evident. It was observed that the use of PCM maintained the air temperature 1-4 °C lower than without using PCM when frequent door opening condition was considered. The highest difference in temperature rise with and without PCM incorporation was observed when door was opened for 10 seconds with an interval of 20 minutes. It decreases with the increase in the duration of door opening. This may be due to more heat load generation with longer duration of door opening. In case of power cut the PCM maintained air temperature 1-4.5 °C lower than without using PCM. The highest difference in temperature rise with and without PCM incorporation was observed when the power failure was for 2 hr. This difference decreases with decrease in duration of power failure. Power saving analysis results showed that highest power saving in door opening condition was observed when door opened for 10 s at 20 min interval. The total energy saved per hour in this case was 1021.88 kJ. In case of power cut for 1 hr the total energy of 3.115 kJ/h was absorbed by PCM which was higher than the rate of energy absorption in other cases which makes it most efficient in this condition. PCM has higher rate of energy absorption in case of 1 h of power failure as compared to others. More power saving will occur when PCM will absorb energy at higher rates so at 1 h of power failure more energy saving will occur than any other situations.
None.
None.

©2018 Raj, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.