MOJ
eISSN: 2381-179X


Case Report Volume 6 Issue 5
1Department of Chest Diseases, Ufuk University Faculty of Medicine, Turkey
2Department of Pathology, Ufuk University Faculty of Medicine, Turkey
3Department of Pathology, Gulhane Training and Research Hospital, Turkey
4Department of Chest Surgery, Ufuk University Faculty of Medicine, Turkey
Correspondence: Nalan Ogan, Department of Chest Diseases, Ufuk University Faculty of Medicine, Turkey, Tel +905052513126
Received: April 27, 2017 | Published: May 2, 2017
Citation: Ogan N, Baha A, Dogan H, et al. Pseudomesotelomatous adenocarcinoma with three cases. MOJ Clin Med Case Rep . 2017;6(5):111-114. DOI: 10.15406/mojcr.2017.06.00174
CT, computerized tomography; FDG, fluorodeoxyglucose; PET-CT, positron emission tomography; VATS, video-assisted thoracic surgery; CEA, carcinoembryogenic antigen; CK-7, cytokeratin-7; NCCN, national comprehensive cancer network
Pseudomesotelomatous adenocarcinoma is a rare, heterogeneous tumor with poor prognosis. Clinical, radiological, cytological and even histopathological findings are similar to epithelial type mesotheliomaIt is difficult to reach the diagnosis with conventional imaging modalities and routine pathologic examination. In this article, 3 cases of our clinic were presented and it was aimed to draw attention to the difficulty and methods of differential diagnosis with mesothelioma.
A sixty-five year-old male patient was admitted to our clinic with complaints of cough and shortness of breath for 7-8months. He was an active smoker for forty-five packets/year, he had not asbestos exposure. 1000cc serohemorrhagic fluid was drained from the patient. No malignant cells were detected on cytological examination. Parasternal emphysema in both lungs, volume loss in right hemithorax and advanced pleural effusion were observed in thorax computerized tomography (CT). Fluorodeoxyglucose (FDG) uptake (SUV max, 7), FDG uptake in several lymph nodes (SUV max, 3.7-4.8) in the right sub paratracheal and subcarinal area of the mediastinum (Figure 1) were present in plaque-like pleural thickening areas be about more pronounced in the middle and lower zones in positron emission tomography (PET-CT) on almost all surfaces of the right hemithorax pleura. Video-assisted thoracic surgery (VATS) was performed with mesothelioma doubt. Tumor showing vascular invasion in places was observed in the form of multifocal small foci and nodulations on visceral and parietal pleura in pathological examination of wedge resection and partial parietal pleuractomy material taken from right lung upper lobe and lower lobe. In immunohincistochemical researches, TTF-1 (thyroid transcription factor-1), CEA (carcinoembryogenic antigen) and CK-7 (cytokeratin-7) were positive,staining with CK20, CK5/6, Melone A, HMB45, WT1 (Wilms tumor) and calretinin was not seen (Figure 2A) (Figure 2B). Intraplasmic mucin staining was detected with PAS-Alcian Blue histochemical staintumor primer was evaluated as lung adenocarcinoma. Progression and pulmonary embolism developed after the 3rd cure in patient who underwent chemotherapy after pleurodesis. The patient died with respiratory insufficiency at the fifth month of follow-up.
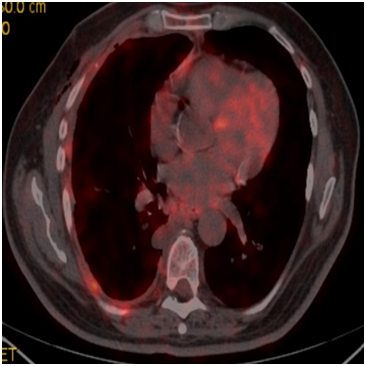
Figure 1 FDG (SUV max:7) uptakes in plaque-like common pleural thickening areas be about more pronounced in the middle and lower zones on almost all surfaces of the right hemithorax pleura.
A sixty-year-old male patient has been placed a drain by thoracic surgeon upon seen massive fluid on the right in the chest x-ray due to shortness of breath. Partial decortication and pleurodesis were performed with VATS on the suspicion of malignancy in fluid cytology. Visceral and mucinous adenocarcinoma infiltration common in the parietal pleura was detected as a result of the biopsy. It was found as WT-1, Calretinin, Thrombomodulin, CK20, CK5/6, D2-40 were negative, TTF-1 was strong, CK7 was strong, CEA was weakly positive (Figure 3A) & (Figure 3B) in immunohistochemical staining. It was referred to our clinicfor treatment arrangements. There were fifteen p-year cigarette stories, he did not drink for 30years, he did not define asbestos exposure. FDG uptake (SUV max, 6-7.6) in the common pleural thickening areas which were evident in the upper and middle zone of the right hemithorax, FDG uptake in the right upper and lower paratracheal conglomerate lymphadenopathies (SUVmax, 7), subcarinal (SUVmax, 6) and left hilar lymph nodes (SUVmax, 3.8) was detected on PET-CT taken (Figure 4). The patient who was scheduled for chemotherapy did not accept the treatment and came out of follow-up.
A seventy-five year old male patient referred to our clinic for dyspnea and involuntary weight loss. He was an active smoker for one hundred thirty packets/year, he had not asbestos exposure. Patient was hospitalized to our clinic for pleural effusion and infection six months ago and no malignancy was detected in Thoracic CT and fluid cytology. The patient whom fluid was lowered with ant biotherapy was discharged at the end of the treatment so as to come to the control. Massive effusion was determined in the left hemithorax in the patient who reapplied on the increase of symptoms. Irregular FDG uptake was present in the areas of common linear nodular thickening in the left hemi thorax pleura on PET/CT (SUV max 7.2-11) (Figure 5). Tumor cells composed of invasive glandular structures in the pleura and with mucinous material in cytoplasm were detected as a result of VATS. The tumor is negative in the pleural fluid. CK8/18, CD15, CEA, CK7 was positive and D2-40, calretinin; WT-1, TTF-1 and CK-20 were negative in immunohistochemical staining (Figure 6). Chemotherapy was started to the patient who was diagnosed with invasive mucinous adenocarcinoma.
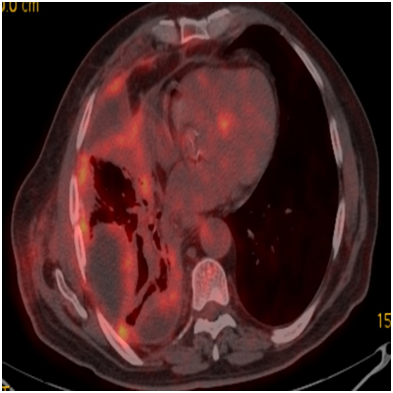
Figure 4 FDG (SUV max 6-7.6) uptakes in diffuse pleural thickening areas evident in the right hemithorax upper and middle zone.
Similarity of lung adenocarcinoma withmalign mesothelioma in terms of radiological, macroscopic and microscopic features but showing immunohistochemical differences are called pseudomesotelomatous adenocarcinoma.1 The incidence was 0.46% in all cancers and 6% in diffuse pleural tumors.2 Pseudomesotelomatous carcinomas are very rare heterogeneous tumors, there are cases reported with other cell types other than adenocancer. All of our cases had mucinous components.3
Clinical features of pseudomesotelomatous adenocarcinoma rare similar to malignant mesothelioma. Lung adenocarcinoma usually occurs in non-smoker female patients with nodular appearance; however it’s mostly male in malign mesothelioma and has an asbestos exposure story. Pseudomesotelomatous adenocarcinoma is commonly occured in males (83-94%), at a later age (mean age 63-68), in smokers (45-87%) and people with asbestos exposure (21-76%).4 Three of our cases were male gender, with an average age of 68.8years, all three cases had smoking history but had no asbestos exposure.
The most common radiological symptoms in pseudomesotelomatic adenocarcinoma are pleural fluid with or without pleural mass, pleural thickening with nodular or irregular surface and all around pleural uptake.5 Most researchers have noted that it is difficult to distinguish with imaging methods such as chest X-ray, CT and magnetic resonance (MR). It is difficult to distinguish primary lung carcinoma or mesothelioma with PET that displays the metabolic characteristic again with the SUV values of the lesions. PET-CT refers to a diffuse pleural tumor and may gained favor in determining the appropriate location for tissue biopsy. It also helps to exclude pleural metastasis from other organ adenocarcinomas.6 In our cases, FDG uptakes was present in several lymph nodes at mediastinum to be more pronounced in middle and lower zones in the common pleural thickening areas where are plaque style, on almost all surfaces of the hemithorax pleura in PET-CT.
Definitive diagnosis is not possible with cytologic evaluation of pleural effusion. VATS is often required to get a sample of the size that is suitable for definite diagnosis.7 Histomorphological, histochemical and immunohistochemical findings should be evaluated together for the definitive diagnosis of pseudomesotelomatic adenocarcinoma. The specificity of TTF-1, CEA and CD15 as the most useful markers in patients with adenocarcinoma is respectively 100%, 97-98.8%, 93% and 67%. TTF-1 is negative and specificity of calretinin is 85-99.8% and specificity of CK5/6 is 83%8 in malign mesothelioma. The results of our cases were consistent with adenocarcinoma.
The correct diagnosis of the disease is important when making a decision about treatment. Chemotherapy with platinum-based dual combinations is the standard approach in mutation-negative patients with lung adenocarcinoma according to the National Comprehensive Cancer Network (NCCN) guidelines. In mutation (EGFR, ALK, ROS1) positive patients, new molecular targeted agents became the standard of first choice treatment. Malignant mesothelioma is a regimen which was performed pemetrexed-platinum therapy activity in the advanced stage. Molecular therapy studies are underway and there is no drug that has shown efficacy for today.9
Clinical results of pseudomesotelomatic adenocarcinoma are not satisfactory because of their resistance to CT and RT treatments.2,3 Average lifetime of non-small cell lung cancer (NSCLC) is 8months similar to stage 4cases.10 The survivor did not receive a response to treatment for our specific single case as a result of survival and 5months of survival could be achieved.
In conclusion, pseudomesotelomatous adenocarcinoma is a rare heterogeneous group of tumors with poor prognosis and can be confused with mesothelioma. True diagnosis is needed for appropriate treatment planning. The differences in the treatment approach are important to evaluate adenocancer cases in terms of new in-use molecular treatment chance although it is not important. Positron emission tomography is advisor in terms of a diagnosis, differential diagnosis and biopsy location. Immunohistochemical examinations are essential for definitive tissue diagnosis.
None.
The author declares no conflict of interest.

©2017 Ogan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.