MOJ
eISSN: 2381-179X


Case Report Volume 5 Issue 2
Department of Oral Medicine and Radiology, Bangalore Institute of Dental Sciences, India
Correspondence: Gaurav, Department of Oral Medicine and Radiology, Bangalore Institute of Dental Sciences, India
Received: October 31, 2016 | Published: November 28, 2016
Citation: Gaurav, Naik V, Sodhi A. Oral erosive lichen planus - a case report with a highlight on etiopathogeneses and immunomodulatory effect of tacrolimus in management of OELP. MOJ Clin Med Case Rep. 2016;5(2):209-212. DOI: 10.15406/mojcr.2016.05.00130
Oral lichen planus (OLP) is one of the most common diseases of the oral mucosa. Clinically, it has specific and clearly identifiable features, bilateral symmetric presentation showing a lace-like network of fine white lines (known as Wickham’s striae) is an essential element of OLP even if the lesion exhibits a mainly atrophic and erosive pattern. Both antigen-specific and non-specific mechanisms may be involved in the pathogenesis of oral lichen planus (OLP).1 The normal oral mucosa may have an overall healthy immune system and any alteration in the immune mechanism could result in OLP and possibly other autoimmune oral mucosal diseases. Recent findings in mucocutaneous graft-versus-host disease, a clinical and histological correlate of lichen planus, suggest the involvement of TNF-a, CD40, Fas, MMPs, and mast cell degranulation in disease pathogenesis. Various treatment modalities are available today in order to treat this lesion with a multifactorial etiology.2
Keywords: oral lichen planus, lichenoid dysplasia, malignant transformation, OLP etiology, conjunctival, rheumatic heart disease
Oral lichen planus is a common chronic inflammatory mucocutaneous disorder that typically affects the oral mucosa and additionally, in some cases the skin. Lichen planus can affect other non-oral mucosal surfaces such as the genitals, anus and pharynx. Conjunctival and oesophageal involvement may rarely occur. Oral lichen planus (OLP) is a T-cell-mediated chronic inflammatory oral mucosal disease of unknown etiology.2,3 Despite intensive research, LP ⁄OLP etiology and treatment remain controversial. It most commonly affects middle aged adults of both sexes, with a slight predominance in women. Skin LP prevalence is unknown Carrozzo et al.,2 but it is estimated to be <1% of the population. LP is thought to be significantly less frequent than exclusive oral LP (OLP) that affects approximately 1–2% of the population. Although the incidence of malignant transformation of OLP remains controversial, careful, regular, and long-term follow-up of patients with OLP is required for the early detection of malignant transformation from OLP. The follow-up interval ranges from 2months to annually.4
A 50years old female patient, (Figure 1) reported to the Department of Oral Medicine and Radiology complaining of severe pain and burning sensation in her mouth for the past two to three months. Her history of presenting illness revealed severe pain and burning sensation in her entire oral cavity which started as a low intensity pain 3years back, it was intermittent in nature and had gradually progressed to the present state for the past 2months. She underwent hysterectomy 20years back followed by disk replacement procedure around 11years back. She was an insomniac for which she was undergoing treatment for the past 15years. She was diagnosed to be suffering from RHD (Rheumatic Heart Disease) and Hyper cholesterolemia 3years back and was on long acting penicillin injections (1 in every 20days) for an year. She had undergone few restorations done in her teeth using silver amalgam. She also had a root canal treatment with crown placement done with respect to a posterior tooth. The patient was unaware of any known drug allergies. The patient had a mixed diet plan and appeared well nourished for her age. Her oral hygiene status was moderate. The patient had no deleterious habits.
On intraoral examination, diffuse lesions in the form of erythematous areas were seen interspersed within white keratotic areas present all over the facial aspect of gingiva (Figure 2) and more posteriorly on the buccal mucosae bilaterally (Figure 3). Left buccal mucosa showed a white erythematous patch measuring approximately 2x2.5cms extending anteroposteriorly from the area adjacent to mandibular first molar to the retro molar pad area and superior inferiorly from the line of occlusion to the upper and lower lingual vestibule. White radiating striae (wickhamstriae) were present with an interspersed erythematous patch. Right buccal mucosa showed a more extensive lesion measuring approximately 2.5x3cms extending anteroposteriorly from the region adjacent to mandibular canine to the retromolar pad area and superioinferiorly from the upper gingiva buccal sulcus to the lower gingivobuccal sulcus and the alveolar mucosa. Borders of the lesion appear irregular and ill defined. On palpation, the lesions appeared to be tender. The surface of the lesions were rough and non scrapable with no induration noted.
On hard tissue examination, all complements of teeth were present in all the 4 quadrants except 46, with generalized attrition and a thick band of local deposits of plaque and calculus. Mobility was absent and occlusion was angle’s class I molar relationship bilaterally. Few carious teeth were also elicited which showed no symptoms like pain, swelling or tenderness. Based on the patient’s history and clinical findings, the lesion was provisionally diagnosed as Oral Erosive Lichen Planus on facial gingivae and buccal mucosae bilaterally.
Differential Diagnosis
Lichenoid drug reactions.Investigations
The histopathological examination of the tissue biopsied revealed it to be case of lichen planus. The hematoxylin and eosin stained section showed epithelium and connective tissue. The epithelium was parakeratinized stratified squamous and hyperplastic in few areas. Few areas also showed atrophic epithelium. Underlying connective tissue showed a dense band of chronic inflammatory cells predominantly lymphocytes along with plasma cells. Prominent blood vessels were seen in the section. Saw tooth rete pegs, which are the hallmarks of lichen planus were clearly evident in the photomicrographs .
Final Diagnosis
Erosive Oral Lichen Planus on facial gingivae and the buccal mucosae bilaterally.Treatment
With the antifungal Candid ointment as the basic regime, topical Tacrolimus 0.1% was prescribed thrice daily for 1month and then the dose was tapered to twice daily for 2weeks after 30days. This was continued for 2months and the patient was monitered continuously for 6months. The patient showed drastic improvement as the lesions had healed completely. She has been put on a periodic recall so that her condition can be monitored and the recurrence of lesion can be prevented.Various white-and-red lesions occur in the oral mucosa, including leukoplakia, erythroplakia, candidiasis, geographic tongue, lichen planus, lichenoid lesions, and others. Oral leukoplakia and oral erythroplakia are well known to be precancerous lesions while the malignant potential of oral lichen planus (OLP) and/or orallichenoid lesions (OLLs) has been the subject of much discussion in the past few decades. Since the clinical and histological features of these white-and-red lesions are similar, differential diagnosis of them is important. Oral lichen planus has a characteristically bilateral distribution, typically involving the buccal mucosa, dorsum and ventral surfaces of the tongue and/or gingiva, when it often presents as desquamative gingivitis.1,2 Palatal and labial involvement is unusual. It is often asymptomatic, although when there are areas of ulceration, the patient experiences varying degrees of discomfort, exacerbated by eating spicy or acidic foods.
There are many antigen-specific mechanisms may be involved in the pathogenesis of OLP, including MHC class I- and MHC class II-restricted antigen presentation by lesional keratinocytes, activation of antigen-specific CD4+ helper T-cells and CD8+ cytotoxic T-cells, clonal expansion of antigen-specific T-cells, and keratinocyte apoptosis triggered by antigen-specific CD8+ cytotoxic T-cells. Apart from there are many non-specific mechanisms may be involved in the pathogenesis of OLP, including (Figure 5) The Heat Shock Proteins (HSPs), reactive oxygen species (ROS), stress, mast cell chemotaxis and degranulation stimulated by T-cell RANTES, endothelial cell adhesion molecule expression stimulated by mast cell TNF-a, T-cell MMP-9 activation by mast cell chymase, epithelial basement membrane disruption by mast cell proteases or T-cell MMP-9, keratinocyte apoptosis triggered by epithelial basement membrane disruption, intra-epithelial CD8+ T-cell migration through basement membrane breaks, inflammatory cell survival prolonged by T-cell RANTES and non-specific T-cell recruitment by keratinocyte-derived chemokines.5,6
OLP can be divided into six types (reticular, papule, plaque, atrophic, erosive, and bullous types), or two types, white and red, while it is most commonly classified into three types, reticular, atrophic, and erosive. Lesions are not homogenous and some cases may present as a mixture of these clinical subtypes. The World Health Organization (WHO) devised a set of diagnostic criteria for OLP in 1978 that was revised in 2003. The modified WHO diagnostic criteria involve differentiation between OLP and OLLs.7,8 In these modified WHO criteria, the essential clinical feature of OLP is defined to be the presence of bilateral lesions that exhibit a lace-like network of white lines(reticular pattern), but not of plaque, atrophic, erosive, and bullous lesions. When the bilateral reticular lesion is absent, then, it is designated as “clinically compatible with OLP”.
Biopsy and histopathological examination of affected tissue may be needed to exclude other diseases that may mimic oral lichen planus , for example discoid lupus erythematosus and to identify possible epithelial dysplasia. The need for biopsy in all cases of suspected lichen planus is debated but it would be appropriate in cases that are atypical in presentation, atrophic or ulcerating. Skin testing for allergy to mercury amalgam may be undertaken where there is a suspicion that there may be a lichenoid reaction in response to this dental material. However there is debate as to the value of such an investigation. The histopathology of OLP was first described by Dubreuill in 1906, and in 1972, it was revised by Shklar who described three characteristic features: overlying keratinization, liquefaction degeneration of the basal cell layer and a dense subepithelial band of lymphocytes.
One of the most important issues concerning OLP is its potential for malignant transformation into OSCC. Although the WHO has categorized OLP as a precancerous condition, the risk of malignant transformation of OLP remains a subject of debate in the literature. It is uncertain what mechanisms could cause malignant trans-formation of OLP. The preferential sites of Oral Squammous Cell Carcinoma (OSCC) which develops from OLP lesions are the tongue and buccal mucosa, and the incidence is higher in the former than the latter, while epithelial dysplasia in OLP is more prevalent in the buccal mucosa.9
The treatment aims of symptomatic oral lichen planus are to heal areas of painful ulceration or blistering. A stepwise approach should be adopted. Topical corticosteroid therapy is the mainstay of treatment for ulcerative disease. There is limited evidence from randomised controlled trials as to the precise efficacy of the various preparations that are in common usage. As an adjunct to therapy, patients should also be advised of the need to maintain a high standard of oral hygiene and any causes of mucosal trauma such as ill fitting dentures, sharp cusps and poor dental restorations should be eliminated. Patients should be informed that there is a very small risk of malignancy associated with oral lichen planus and that long term monitoring is appropriate.10
In our case, methodical and systematic use of Tacrolimus (immunomodulator) showed a drastic improvement in healing of the persisting lesions completely. Tacrolimus is a macrolide calcineurin inhibitor. Calcineurin is a calcium and calmodulin dependent serine/threonine protein phosphatase which activates the T-cells of the immune system. When the activation of T cell receptor occurs, there is increase in the intracellular calcium which in the presence of calmodulin as a catalyst activates calcineurin. This step is followed by “de phosphorylation” that stimulates the movement of Transcription factor of Nuclear factor of activated T cells to the nucleus of T cell, thereby increasing the activity of genes coding for IL-2 and other cytokines. This has been found to be one of the mechanisms resulting in OLP like lesions. In this disease mechanism, TACROLIMUS acts at the dephosphorylation stage (Figure 6) thus obstructing it and bringing about phosphorylation. This ultimately decreases the activity of genes coding for various ILs which ceases the progress towards OLP like lesions. This has been pictorially represented below.11,12
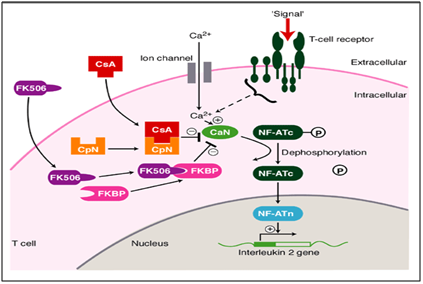
Figure 6 Mechanism of action of tacrolimus as an immunomodulator in treatment of oral erosive lichen planus.
Various studies have found topical Tacrolimus to be effective in the treatment of OLP and some have also reported a better initial therapeutic response than other drugs including the corticosteroids. But studies evaluating the efficacy of topical Tacrolimus in Indian population especially over long periods, are scarce. Tacrolimus (0.1%w/v) has been reported to be effective and safe for the treatment of OLP by various investigators. It has been found to be an effective means of controlling the symptoms and signs of erosive or ulcerative oral lichen planus and had no notable adverse effects over a mean duration of application of 19.8 months.13 In a meta analysis by Chamani G et al.,14 it was concluded that topical Tacrolimus is an effective alternate to various corticosteroids and may be considered as a first line therapy in the management of OLEP.
Currently, as the cause of lichen planus remains unknown, there are no specific preventative regimes for this disorder. The pathogenesis of OLP may involve both antigen-specific and non-specific mechanisms. However, regular clinical review is deemed prudent in view of the controversy regarding the malignant potential of this condition. The diagnosis of oral lichen planus is initially based upon the clinical presentation of bilateral white patches with or without ulcers or blisters, typically affecting the buccal mucosa, ventral, lateral and dorsal surfaces of the tongue and gingiva. ‘NICE’ guideline 27 [Referral Guidelines for Suspected Cancer] clearly states that patients with oral lichen planus should be monitored for oral cancer as part of the routine dental examination. Clearly, more work is required for a full understanding of the etiology and pathogenesis of OLP.1,2,5,11
None.
The author declares no conflict of interest.

©2016 Gaurav,, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.