MOJ
eISSN: 2381-179X


Case Report Volume 10 Issue 6
1Department of Medicine, Tufts Medical Center, USA
2Deparment of Radiology, Tufts Medical Center, USA
3Department of Pathology, University of Massachusetts Medical School, Massachusetts General Hospital, USA
4Department of Pathology, Newton-Wellesley Hospital, USA
5Department of Medicine, Division of Hematology/Oncology, Tufts Medical Center, USA
Correspondence: Danai Dima, Deparment of Radiology, Tufts Medical Center, Boston, MA, 02111, USA, Tel +1 (312)-316-8567
Received: November 19, 2020 | Published: November 27, 2020
Citation: Zhang D, Dima D, Hu X, et al. NRAS Q61R-mutant mucosal melanoma with cardiac metastasis - a diagnostic challenge. MOJ Clin Med Case Rep. 2020;10(6):146-150. DOI: 10.15406/mojcr.2020.10.00365
We present this case report of a patient with NRAS Q61R-mutant melanoma, suspected to be mucosal melanoma of small bowel origin with cardiac, hepatic and pulmonary metastases. We focus on the unique manner of diagnosis (requiring the finding of a specific molecular signature via liquid biopsy in conjunction with tissue biopsy) and management of this rare cancer with first-line immunotherapy with dual checkpoint blockade followed by maintenance PD-1 inhibition, allowing the patient to have sustained clinical response at eight months since original diagnosis.
Keywords mucosal melanoma, NRAS Q61R-mutant melanoma, liquid biopsy, immunotherapy
Mucosal melanoma (MM) is a rare cancer originating from extra-cutaneous melanocytes located in mucosal membranes lining the respiratory, gastrointestinal and urogenital tracts. It accounts for approximately 1.4% of all melanoma diagnoses, with an incidence rate of 2.2 cases per million per year in the United States.1–3 Moreover, compared with cutaneous (80%) and ocular melanomas (74%), mucosal melanoma have the lowest five-year survival of 25%.3
Primary mucosal melanoma commonly arises from anorectal (31% anal canal, 22% rectum) and oropharyngeal (33%) sites, while small bowel (2.3%) is a rare site of origin.3 A finding of melanoma in the small bowel would therefore prompt investigation of whether this represents metastatic disease versus the less common primary mucosal melanoma as this may affect prognosis and treatment strategies. Furthermore, although cardiac metastases may develop from mucosal melanoma, data on patients treated for such a presentation are minimal, particularly as cardiac metastases are rarely diagnosed in the ante-mortem setting.4,5
Common oncogenic “driver” mutations in melanoma include BRAF, NRAS, and KIT , which are present in different frequencies in cutaneous versus mucosal melanoma.6–7 While a targetable driver mutation in codon V600 of the BRAF gene occurs in approximately 50% of cutaneous melanomas, the incidence is only 3-15% in mucosal melanomas.8 In contrast, KIT mutations tend to be rare in cutaneous melanomas but occurs in 10-22%% of mucosal melanomas.7 Activating NRAS mutations occur in over 20% of cutaneous melanoma but only in around 10% of mucosal melanoma, and confers poor prognosis.9
We present a patient with NRAS-mutant melanoma, suspected to be mucosal melanoma of small bowel origin with cardiac hepatic and pulmonary metastases, focusing on the unique manner of diagnosis and management of this rare cancer.
A 62-year-old Caucasian woman was admitted to the hospital with three months of worsened dyspneaon exertion, fatigue and unintentional weight loss of ten pounds. Labs showed iron deficiency anemia with hemoglobin down trended to 6.4g/dL and positive fecal occult blood test, normal CEA (1.5ng/mL) and CA 19-9 (7U/mL), with elevated LDH (541IU/L).CT scan of the chest, abdomen and pelvis revealed two non-obstructing small bowel masses(5.3cm jejunal mass and 12.3cm ileal mass), hypoattenuated right hepatic lesions, sub-centimeter right pulmonary nodules (up to 9mm), and a 3cm by 3cm by 4cm intracardiac mass at the tricuspid annulus(Figures 1A, 1C, 2A). MR of the liver revealed three right hepatic lobe lesions, measuring up to 1.3cm. Transthoracic echocardiogram confirmed the large mobile right atrial mass attaching below the anterior tricuspid valve leaflet and protruding into the right ventricle (Figure 3). Subsequent cardiac MRI characterized the right atrial mass as a mixture of tumor and thrombus. MRI brain revealed small subacute infarct in the left cerebellar hemisphere, but no intracranial metastasis.

Figure 1 Computed tomography scans of the abdomen/pelvis demonstrate treatment response to immunotherapy with 4 cycles of ipilimumab/nivolumab followed by maintenance PD-1 inhibition. The arrows point to the large ileal melanoma lesion, at time of diagnosis (A, C) and at 6 months after (B, D).
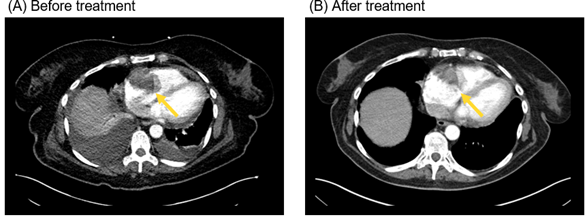
Figure 2 Computed tomography scans of the chest demonstrate treatment response to immunotherapy with 4 cycles of ipilimumab/nivolumab followed by maintenance PD-1 inhibition. The arrows point to the right atrial melanoma lesion at time of diagnosis (A) and at 6 months after (B) immunotherapy was given.
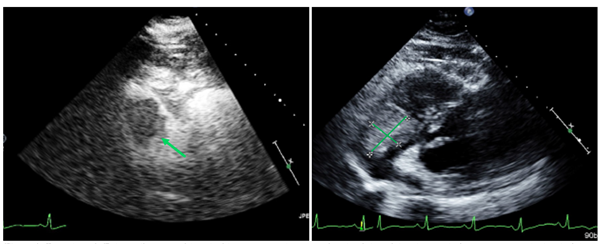
Figure 3 Transthoracic echocardiogram with and without contrast demonstrates the right atrial melanoma lesion before treatment at the level of the tricuspid valve.
CT-guided biopsy of the large ileal mass revealed a high grade, poorly differentiated malignancy with high mitotic activity and extensive necrosis (Figure 4). Immunohistochemistry (IHC) was positive for vimentin, S100 (focally), and SOX10 (diffusely), with preserved H3K27me3 expression indicating that this is not a malignant peripheral nerve sheath tumor (Figure 5). Negativity of all epithelial (CK7, CK20, AE1/AE3, pan-cytokeratin OSCAR, MNF116), neuroendocrine (chromogranin, synaptophysin), lymphocytic (CD45), vascular (CD31, CD34), and other mesenchymal (CD34, CD117, desmin, myoD1, myogenin, CD99) markers eliminated these potential tumor origins from the differential. IHC was negative for BRAF-V600E and the melanocytic markers (HMB45, Mart-1, Melan-A). Altogether, this immunoprofile remained nonspecific, being most consistent with either melanoma or clear cell sarcoma-like tumor of the gastrointestinal tract (CCSLGT). Tumor cells were negative for EWSR1 rearrangement (associated with CCSLGT) by fluorescence in situ hybridization.
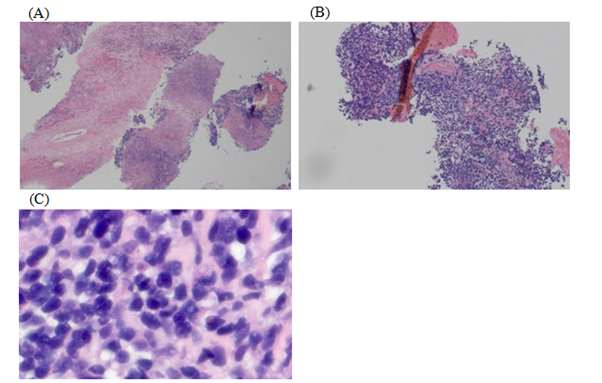
Figure 4 The H&E stain demonstrates the poorly differentiated nature of this malignancy. Low power H&E sections (A and B) show a diffuse proliferation of tumor cells in a background of extensive necrosis. High power H&E section (C) shows tumor cells that are intermediate in size and display scant cytoplasm, relatively homogenous chromatin, and inconspicuous nucleoli.

Figure 5 Tumor cells from the ileal biopsy show retained H3K27me3 expression (A) and are positive for S100 (B) and SOX10 (C) on immunohistochemistry.
While awaiting final pathologic diagnosis, the patient became transfusion-dependent from persistent bleeding of the small bowel tumors. A multidisciplinary team deemed the risk of surgical complications and death to be prohibitively high, given the potential for cardiac outflow obstruction during induction anesthesia. Surgical intervention including resection of the small bowel masses and/or removal of the cardiac mass was not recommended. Hospitalization was complicated by respiratory failure from bilateral pulmonary emboli and heparin-induced thrombocytopenia. Attempts to obtain additional tissue biopsy were limited by these complications, the difficult location and accessibility of her cardiac mass, and high risk of bleeding from procedures. CT-guided biopsy of a suspected hepatic metastasis was non-diagnostic and caused significant post-procedure pain. Liquid biopsy was then obtained via Guardant360 assay, revealing 4.4% of cell-free tumor DNA harboring NRAS Q61R, a mutation commonly seen in melanoma, but rarely in sarcoma. Taking into account the clinical presentation, histologic evaluation, and liquid biopsy finding, the diagnosis was rendered metastatic NRAS Q61R-mutant melanoma, suspected to be mucosal melanoma from small bowel origin.
Dual checkpoint blockade with ipilimumab (3mg/kg) and nivolumab (1mg/kg) given intravenously was started on hospital day 18 with good tolerance. The patient was discharged after 40 days of hospitalization, with continued requirement for intermittent blood transfusions. Molecular testing was confirmed and expanded on via FoundationOne analysis of the original tissue biopsy, revealing tumor mutational burden of 14 mutations/Mb, homozygous loss of CDKN2A and CDKN2B, and TP53 1245S in addition to NRAS Q61R and MSI stable status. She proceeded to complete four cycles of ipilimumab/nivolumab dosed every three weeks, followed by maintenance PD-1 inhibitor, and improved clinically with less frequent blood transfusion requirement. At six months since diagnosis, updated scans and echocardiogram continued to show treatment response with stable to reduced sizes of the cardiac and mesenteric masses (Figure 1B, 1D, 2B).
While primary melanoma of the small bowel is exceedingly rare, the small bowel is the most common site involved by metastatic gastrointestinal melanoma.3 In a retrospective study, Elsayed et al. have suggested that most if not all melanomas involving the small bowel represent metastases from unknown or regressed primary cutaneous melanoma.10 Thus, it is crucial to exclude primary cutaneous or ocular melanoma at the time of presentation. If primary mucosal melanoma of the small bowel is a true entity, its pathogenesis is poorly understood, especially as melanocytes have not been demonstrated in the small bowel. One hypothesis is that melanoblasts migrating to the distal ileum via the omphalomesenteric canal may be what primary melanoma of the small bowel derives from. While it is possible that our patient may have had a regressed primary cutaneous melanoma with resultant metastatic presentation to the small bowel, her clinical history and exam suggested otherwise. Given the large size of the small intestine tumors, this was suspected to be the primary lesion.
Diagnosis was the biggest challenge in this particular case. Tissue biopsy from the ileal mass had narrowed down the differential but was not definitive, and obtaining another tissue biopsy would have been challenging given her complicated clinical course and location of her masses. A non-invasive liquid biopsy was therefore utilized and instrumental in supplying enough missing molecular information to solidify diagnosis prior to initiating appropriate treatment.
Liquid biopsies detect circulating cell-free (cfDNA), small fragments of DNA that are shed with rapid cell turnover in plasma or other body fluid (urine, saliva, cerebrospinal liquid).11 Circulating tumor DNA (ctDNA) are the subset of cfDNA shed from tumor cells. These fragments can represent the entire genome of a cancer in real time including the primary lesion and metastases, whereas tissue biopsy provides only a snapshot of the tumor limited to the particular site sampled. Liquid biopsy can therefore reflect tumor heterogeneity more effectively than tissue biopsy in certain situations.12 Efficient extraction of a malignancy’s molecular signature via liquid biopsy, a relatively non-invasive test, can complement tissue biopsy to solidify diagnosis and prevent delays in therapy initiation. This can be especially useful in patients presenting with poorly accessible or small tumors or for whom tissue biopsy confers risk of complications.
Liquid biopsy has implications in each stage of cancer management: diagnosis, screening, detecting recurrence, and checking for necessity for adjuvant therapy. Several studies have been investigating its utility as an alternative screening tool for colorectal cancer (CRC). An interesting observation was that the average cfDNA concentration in the serum of individuals with metastatic CRC was 5times higher than healthy individuals.13 Overall, there have not been many studies exploring the use of liquid biopsy as a diagnostic tool for melanoma or other unidentified primaries.
In metastatic melanoma, the most well studied ctDNA biomarker via liquid biopsy is the BRAF-V6000 mutation. Monitoring BRAF-V6000 levels could help predict prognosis and response to therapy. Several trials showed that high ctDNA levels prior to Dabrafinib treatment were associated with lower response rates and progression free survival. Also, ctDNA levels were correlated with overall tumor burden.14 Gray et al.,15 showed that increase in ctDNA can precede radiological evidence of progression. Furthermore, detection of ctDNA with an NRAS-mutation was associated with development of resistance to BRAF inhibitors, which could help determine timing for therapy switch.15 Despite these promising advantages, ctDNA is underutilized in the clinical management of metastatic melanoma. Larger trials should be pursued to corroborate these findings. In our case we used Guardant-360, which is a next-generation sequencing product able to evaluate 73 genes. This product was originally created to identify targetable mutations in newly diagnosed metastatic non-small-cell lung cancer and is now used in several solid tumors to guide molecular therapy.16 However, as our case has shown, it can potentially have utility in diagnosis as well.
Detection of NRAS Q61R mutation via liquid biopsy expedited the diagnosis of melanoma in our patient. NRAS encodes a member of the RAS family of small GTPases that mediate transduction of growth signals. Activation of RAS signaling causes cell growth, differentiation, and survival by activating the RAF-MAPK-ERK, pI3K, and other pathways.17 NRAS alterations affecting various amino acids including Q61have been characterized to be activating and oncogenic.17 In one series, NRAS mutations were present in 20% of cutaneous melanomas and 12% of mucosal melanomas of which 54% were located on Q61.The most commonly observed NRAS codon 61 mutations are the Q61R and Q61K changes.7 Therefore, taking into account our patient’s clinical presentation, the available tissue pathology, and the molecular profile from the liquid biopsy result, her diagnosis was deemed to be metastatic NRAS-mutant melanoma, suspected to be mucosal melanoma from small bowel origin, also involving the heart and liver. Additional molecular testing in this patient revealed homozygous loss of CDKN2A and CDKN2B, further supporting the diagnosis of melanoma. Homozygous deletion of CDKN2A and/or CDKN2B has been reported in 14-29% of melanoma.18
When feasible, surgery is the primary treatment modality for mucosal melanoma, although prognosis remains limited with median survival of a little over a year.19 Literature cites five-year survival rate for mucosal melanoma of 25% or lower, significantly poorer compared to rates for cutaneous and ocular melanoma.20 In the setting of cardiac metastasis, prognosis is usually worse, as this signifies presentation with very advanced disease. A retrospective study by Zitzelsberger et al found that median survival after the detection of cardiac metastasis was 8 months. Past studies have reported five-year survival rate for metastatic melanoma involving the heart to be generally less than 20%. More recently, five-year survival in those with cardiac metastasis has improved to 37% with the advent of targeted therapies and immunotherapy.21
Prospective data are limited regarding systemic treatment of metastatic mucosal melanoma, with no randomized controlled trials available. In the real-world setting, therapy is extrapolated from the well-established guidelines of cutaneous melanoma. Immunotherapy with anti-PD-1 antibodies is the standard treatment for metastatic cutaneous melanoma, as outcomes are better compared to ipilimumab or chemotherapy.22–26 A post-hoc analysis of three clinical trials, including a more general melanoma population, revealed that pembrolizumab provides durable antitumor activity in the mucosal melanoma subgroup.27 It also showed that mucosal melanoma tends to have weaker response to PD-1 inhibitor monotherapy compared with cutaneous melanoma, raising the question of potential benefit from dual checkpoint blockade in mucosal melanoma. The increased efficacy of dual checkpoint blockade was also supported by a combined analysis of 6 studies, in which patients with mucosal melanoma treated with ipilimumab/nivolumab had better response and survival benefit compared to those treated with nivolumab alone.28 Several other small retrospective studies and case series have noted the benefit of immunotherapy. Several other small retrospectives studies and case series have also observed the benefit of immunotherapy. An ongoing trial is exploring the use of nivolumab/ipilimumab in metastatic mucosal melanoma and assessing for potential biomarkers of response to immunotherapy (NCT02978443).
Patients with mucosal melanoma harboring BRAF and KIT mutations, can benefit from targeted therapies. Interestingly, our patient sustained NRAS Q61R mutation, which remains a focus of much ongoing research, as no effective molecularly targeted inhibitors have yet been approved for its treatment. The NRASQ61R-mutant melanoma is typically more aggressive and associated with poor outcomes. Immunotherapy has been shown to be effective in NRAS-mutant melanoma, however its role in the management of mucosal melanoma is not as well understood. A retrospective study by Johnson et al.,29 showed that patients with NRAS-mutant melanoma, compared to those who are NRAS-wild type, have higher response rates to immunotherapy, especially in the first-line setting (28% versus 16%) and superior clinical benefit rate when PD-1/PD-L1 inhibition is given (73% versus 43%), with non-statistically significant higher PD-L1 expression in NRAS-mutant melanoma compared to other genotypes (50% versus 30% with ≥5% expression).29 Prospective data are needed to confirm the efficacy of immunotherapy in NRAS-mutant melanoma. Promising targeted therapy for NRAS Q61-mutant melanoma includes MEK inhibitors such as binimetinib, which improved progression-free survival compared with dacarbazine (2.8 versus 1.5 months, HR 0.62) in the NEMO trial (NCT01763164).30
This case report highlights the complementary use of liquid biopsy, a relatively non-invasive test, along with tissue biopsy in expediting a diagnosis of melanoma and establishing its molecular signature, which would not have been possible with tissue biopsy alone. Despite poor prognosis on presentation with unresectable NRAS Q61R-mutant melanoma, suspected to be a rare primary mucosal small bowel melanoma, with cardiac metastasis, this patient responded favorably to upfront dual checkpoint blockade transitioned to maintenance PD-1 inhibition. Although the duration of her response has yet to be determined, we are encouraged by her experience of partial tumor shrinkage with stabilization of clinical course over eight months thus far, with intensive supportive management.
None.
None.
The authors have no conflicts of interest to declare.

©2020 Zhang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.