MOJ
eISSN: 2381-179X


Case Report Volume 7 Issue 5
1Department of Internal Medicine, Cerrahpasa Medical Faculty, Istanbul University, Turkey
2Department of Pulmonary Medicine, Cerrahpasa Medical Faculty, Istanbul University, Turkey
Correspondence: Cuneyt Tetikkurt, Department of Internal Medicine, Cerrahpasa Medical Faculty, Istanbul University, Turkey, Tel 90-216-360 19 77, Fax 0-212-587 02 17
Received: November 14, 2017 | Published: November 28, 2017
Citation: Bilir M, Tetikkurt C, Yanardag H. Miliary tuberculosis occuring during certolizumab treatment. MOJ Clin Med Case Rep. 2017;7(5):301-303. DOI: 10.15406/mojcr.2017.07.00218
Tumor necrosis factor (TNF) alpha inhibitors play an important role in the treatment of immune mediated diseases including rheumatoid arthritis, the seronegative spondyloarthropaties, psoriasis, and inflammatory bowel disease. These agents have significant potential for adverse effects that lead to reactivation and dissemination of latent tuberculosis infection. We present a patient with miliary tuberculosis occuring during treatment with certolizumab for ankylozing spondylitis under INH prophylaxis.
A 57 old female patient presented with dry cough, fever, loss of apetite, and chest pain present for three weeks. Chest x-ray showed diffuse miliary nodules andright pleural effusion. Computed tomography revealed diffuse miliary nodules, infiltration in the right lower lobe, and right pleural effusion. Pleural effusion was exudative in character and had a 74% lymhocyte ratio with a high level (114U/L) of adenosine delaminate. Sputum smear was positive for acid-fast bacilli and mycobactrium tubeculosisgrew in culture. The diagnosis was miliary tuberculosis associated with certolizumab treatment. Four weeks after with antituberculous drug treatment the symptoms completely resolved while the radiologic lesions diminished significantly.
TNF alpha inhibitors are potent anti-inflammatory agents. Alpha inhibition may result in severe complications and adverse effects. Clinicians should bear in mind that severe immunosupression leading to mycobacterial infection may come out even if the patient is undera prophylactic treatment for tuberculosis and current screening methods for latent tuberculosis may be inadequate to identify the latent infection.
Keywords: certolizumab, miliayr tuberculosis, TNF-alfa antagonists, tuberculosis
TNF-alpha inhibitors serve as important treatment options for a variety of immune mediated diseases with a major impact on the treatment of disabling inflammatory disorders. These agents submit a targeted strategy that contrasts with the nonspecific traditional immunosuppressive agents. However, significant complications and severe adverse effects may occur during treatment with these targeted TNF-alpha inhibitor drugs. One of the important side effect of TNF-alpha blockers is increased risk for reactivation of latent tuberculosis and dissemination of tuberculosis infection.1–4
Screening and identification of tuberculosis infection may be challenging and troublesome in patients treated with the TNF-alpha antagonists. Although screening for latent tuberculosis is routinely performed before treatment with these agents, de novo tuberculosis infection may come out even under prophylactic treatment.
We present a patient under isoniazid prophylaxis in whom certolizumab treatment led to dissemination of latent tuberculosis infection resulting in miliary tuberculosis. This case report illustrates the clinical hazards and complications1,4,5 associated with tuberculosis that emerged during anti-TNF-α treatment. Current surveying tools for latent tuberculosis6–9 may be inconclusive and thereby significant consequences of pulmonary tuberculosis like miliary dissemination cannot be prevented by using the prevailing laboratory techniques in some cases. Patients may develop de novo miliary disease even if they had received prophylaxis against latent infection. Routine screening for latent tuberculosis is not reliable to preclude the serious complications of latent tuberculosis.
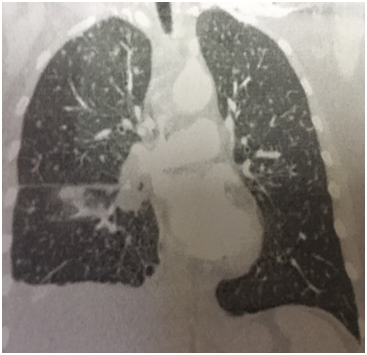
A 57 year old Caucasian female was admitted for dry cough, fever, loss of appetite and chest pain for three weeks. She had a history of tonsillectomy, ankylozing spondylitis, uveitis, pelvis fracture and tibia fracture. Her father died of colonic carcinoma. Her mother had hypertension and previous pulmonary tuberculosis. The patient was under treatment with certolizumab, methotrexate, and prednisolonefor ankylozing spondylitis and uveitis. Daily 300mg isoniazid was also given simultaneously with certolizumab for prophylaxis. Initial laboratory findings revealed WBC 8.2X103/mm3, hemoglobin 10.8g/dl, platelets 341X103mm3, lymphocytes 1.4X103/mm3, creatinine 0.74mg/dl, AST 18IU/L, ALT 18IU/Lmm3, LDH 167IU/L, albumine 3.56gr/dl, CRP 18.6 mg/dl, and calcium 9.1 mg/dl. ECG showed sinus ryhtm. Tuberculin test was negative. Chest x-rayshowed diffuse miliary nodules, alveolar infiltration in the right lower lobe, and right pleural effusion (Figure 1). Pleural protein 4.57g/dl, LDH 353U/L, and albumin 3.56g/dl. Pleural fluid had 1540cells/mm3 with a 74% lymphocyte ratio. Pleural fluid ADA was 114U/L (normal 0-40 U/L). The pleural fluid was exudative compatible with tuberculosis. Computed tomography of the thorax revealed diffuse miliary nodules, infiltration in the right anterior segment of the lower lobe, and right pleural effusion (Figures 2-4). Sputum stains was positive for acid-fast bacilli. Mycobacterium tuberculosis was isolated from the sputum culture. The final diagnosis was miliary tuberculosis associated with certolizumab occuring on the third month of treatment. The patient was commenced on pyrazinamide, isoniazid, rifampicine, and ethambutol treatment for tuberculosis while certolizumab treatment was stopped.
TNF-alpha-antagonists are remarkably effective agents in the treatment of various immune mediated diseases like rheumatoid arthiritis, inflammatory bowel disease, and psoriasis.10–13 TNF-alpha constitutes an important role of defence against tuberculosis and mechanisms of anti-TNF-α agents lead to tuberculosis by impairing tuberculosis immune response.14,15 This case report illustrates the various clinical pitfalls and complications that can be encountered during anti-TNF-α treatment.
The patient had a negative tuberculin test and a normal chest x-ray before certolizumabtreatment. She was commenced on prophylactic isoniazid treatment because the patient had an exposure to active tuberculosis. Miliary tuberculosis with pleural effusion and right lower lobe infiltration occured on the third month of treatment. The patient had an appropriate screening for tuberculosis including medical history, tuberculine test, and chest x-ray before anti-TNF-α treatment was started. The sensitivity of the tuberculine test may have been restricted or diminished by the previous immunosuppressive treatment in our patient. The normal chest x-ray before treatment had also a low diagnostic yield for revealing sequela of past or current infection. Following treatment the right lower anterior segment infiltration was only identified at the computed tomography coronal image and was not detected in chest x-ray.
Our case shows the inadequacy of current screening tools for tuberculosis before commencing anti-TNF-α agents. Second, a serious complication like miliary tuberculosis developed in this patient while the patient was under prophylactic isoniazid regimen. And as far as we know, this is the first case of miliary tuberculosis occurring in association with certolizumab treatment.
The role of TNF-α associated with the immune defence mechanisms against tuberculosis is not clear. The pathologic contrivance produced by the anti-TNF-α agents is not explicit either. Thereby, the current screening tools including patient history, tuberculine test, and chest x-ray for latent tuberculosis are appearently incompetent and unreliable for identifying the patients at risk who are prone to develop tuberculosis or its complications associated with TNF-alpha anatgonists.
We would like to acknowledge Khumphan Amaratana for his contribution toward the revision of the manuscript.
The author declares no conflict of interest.

©2017 Bilir, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.