MOJ
eISSN: 2381-179X


Case Report Volume 2 Issue 1
1Department of Surgery, Prince of Wales Hospital, Australia
2Department of Anatomical Pathology, Prince of Wales Hospital, Australia
Correspondence: Francis Ting, Department of Surgery, Prince of Wales Public Hospital, Barker Street, Randwick NSW 2031 Australia, Tel 61410809618
Received: January 18, 2015 | Published: March 16, 2015
Citation: Ting F, Smith R, Davidson T, et al. Low grade myoepithelial carcinoma: a mini case series analysis including a case with sacral disease and a case with forearm disease. MOJ Clin Med Case Rep. 2015;2(1):18-22. DOI: 10.15406/mojcr.2015.02.00012
Myoepithelial (ME) tumours of soft tissue are rare tumours that were first described in 1995. A wide spectrum of architectural and histological patterns make diagnosis difficult; as is differentiating benign or low grade from malignant or high grade tumours to guide treatment and predict patient outcome. We report two cases of low grade ME tumours of soft tissue treated at our institution in 2013. The first one was in a 24year old previously healthy male who presented with three separate deposits of tumour. The two largest were a sacral mass measuring 8x12x21cm and left inguinal mass measuring 8.6x10x8cm. He was successfully treated with neoadjuvant chemotherapy and wide local excision with flap reconstruction. The second case was in a 35year old female who presented with a slowly growing right forearm mass. Due to positive margins she underwent three excisions and post operative brachytherapy for recurrence prevention. Both patients are disease free at 6 month follow up. A review of the current literature surrounding presentation, epidemiology, histopathological findings and management of ME tumours of soft tissue is correlated with findings from this mini case series analysis.
Keywords: myoepithelioma, neoplasms
Myoepithelial tumours of soft tissue (termed myoepitheliomas or myoepithelial carcinomas), as distinct from their salivary gland counterparts, are rare tumours that were first described in 1995.1 A wide spectrum of architectural and histological patterns make diagnosis difficult; as is differentiating benign or low grade from malignant or high grade tumours to guide treatment and predict patient outcome.
We describe 2 cases treated at our institution in 2013, followed by a review of the literature, to add to the limited pool of data available on this poorly described group of heterogeneous tumours.
Case 1
A 24year old previously healthy male was referred to our tertiary institution with a left sacral (Figure 1) and left groin mass (Figure 2). He reported a slowly enlarging subcutaneous left sacral swelling for the 2years prior to referral, followed by a swelling in his left groin appearing 12months after this. During this time he reports having no bowel, urinary or constitutional symptoms, however some discomfort from the groin mass was reported with sitting down. Incredibly the patient (and his partner) waited 2years, until the mass had grown to a conspicuous size, before seeking medical attention. A positron emission tomography computed tomography (PET CT) was performed which confirmed the clinical findings. It revealed a large mass posterior to the sacrum extending down to the proximal left thigh measuring 8x12x21cm (H), a large mass in the left inguinal region measuring 8.6x10x8cm (H), and a mass lateral to the left gracilis measuring 2.8cm. All three masses demonstrated abnormal tracer uptake and were reported as having central necrosis or cystic degeneration. A 2cm left internal iliac lymph node (mildly FDG avid) was reported as suggestive of tumour involvement, and a 1cm right internal inguinal and 14mm pelvic wall node was identified. Core biopsy performed at this time was reported as favouring extra-skeletal myxoid chondrosarcoma. Due to the proximity of the tumour to his anus, the patient underwent laparoscopic assisted formation of loop ileostomy brought out to his right upper quadrant, away from the field of neoadjuvant radiotherapy, to decrease the perineal contamination associated with the definitive surgery and also with the side effects of radiotherapy. A 2week course of neoadjuvant Eilber chemoradiotherapy using Adriamycin as a radio sensitiser for intensity modulated radiotherapy (IMRT) to a total dose of 30Gy in 18 fractions was administered. Follow up CT scan revealed slight decrease in the size of the pelvic masses, however the pelvic lymph nodes had remained unchanged and the pelvic wall node had increased from 14 to 18mm in size.
Definitive operation was performed 6 weeks after commencing neoadjuvant chemoradiotherapy. Excision of the lateral thigh, left groin and sacral masses was performed. Adductor longus fascia and sartorius fascia was removed in continuity with the left groin mass, and a wide excision of the buttock and sacral mass performed, with gluteal fascia and further excision of left gluteal muscles removed as a margin (Figure 3). Dissection was continued onto sacrum down to coccyx, with some pubococcygeus taken, with preservation of sphincters and rectum. Excision of lymph nodes from pelvic, internal and external iliac groups was performed and flap reconstruction performed by the plastic surgery team at the end for closure (Figure 4).
Histopathology came back as consistent with low grade myoepithelial (ME) carcinoma with clear margins for all 3 masses, with the lateral thigh mass measuring 33x70x28mm, the left groin mass measuring 85x150x90mm and the sacral/buttock mass measuring 80x190x60mm. 2 left groin lymph nodes, 7 external iliac lymph nodes and 6 left pelvic nodes were examined and all were negative for tumour. Microscopically, sections showed acinar type or papillary proliferation of plump epithelioid cells with palely eosinophilic or clear cytoplasm and relatively uniform and only mildly atypical vesicular nuclei (Figures 5) (Figure 6). It was well circumscribed with a pseudo capsule with extensive areas of necrosis. Immunostaining came back strongly positive for S100 protein, GFAP, with scattered cell positivity for EMA and rare cells positive for keratin CAM 5.2. Staining for pan keratin, p63, TFE-2 and Olig 2 were negative. Staining for INI-1 was positive.
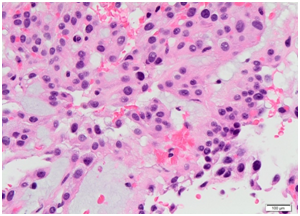
Figure 5 Histopathological slide at high power for case 1 showing mildly atypical myoepithelial cells with finely vacuolated eosinophilic cytoplasm containing myxoid matrix.
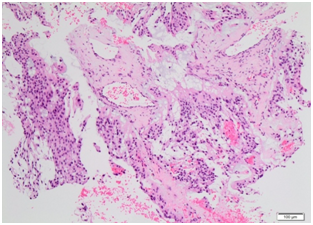
Figure 6Histopathological slide at low power for case 1 showing moderately cellular lobulated tumour, composed of sheets of mildly atypical epithelioid myoepithelial cells with abundant eosinophilic cytoplasm and interspersed myxoid matrix.
The patient’s loop ileostomy was reversed without complication at 4 months post operatively and heis now clinically well at 6months post op.
Case 2
A 35year old female was referred to our tertiary institution with a slowly growing right forearm mass which she had first noticed 14months previously. She was a non smoker with a history of thyroidectomy for thyrotoxicosis. The patient reported increasing local discomfort and pain affecting her ability to work as a hair dresser. Magnetic resonance imaging (MRI) performed showed a contrast enhancing well circumscribed mass measuring 24x13x12mm within the brachioradialis just below the elbow joint reported as being suggestive of a schwannoma. Core biopsy was reported as consistent with a benign spindle cell tumour; probably a schwannoma and she had undergone excision of this mass 2months before referral.
Histopathology from this initial resection showed a multilobulated neoplasm composed of spindle shaped or more ovoid cells, distributed in a lobular fashion within a prominent myxoid matrix (Figures 7) (Figure 8). The lesion was unencapsulated, with the bulk of the cells having palelyeosinophilic indistinct cytoplasm and plump mildly atypical vesicular nuclei with small nucleoli. Immunostaining showed multifocal strong positivity for S-100 protein and EMA along with focal positivity for keratin CAM 5.2 and focal weak positivity for calponin, whilst staining for pan-keratin, GFAP, p63 and AE1/AE3 were negative. The findings were consistent with low grade myoepithelial carcinoma, involving excision margins.
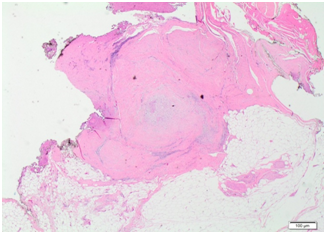
Figure 7Histopathological slide at low power for case 2 showing fibrocellular scar tissue containing microscopic focus of residual tumour.
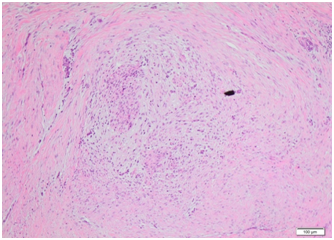
Figure 8Histopathological slide at high power for case 2 showing microscopic focus of residual tumour composed of spindled myoepithelial cells within fibromyxoid stroma.
Re excision of the sarcoma was performed 4 months after the initial resection, with the old incision excised and extended. Brachioradialis was dissected out, radial and cutaneous nerves were identified and protected, and scarring from the previous excision was found to be in the crevice between brachioradialis (BR) and extensor carpi radialis (ECR). The tumour was unable to be palpated, but the scar tissue was excised, with a 4cm margin of muscle taken at this border between BR and ECR. The specimen taken measured 60x13x17mm deep. Histopathology showed residual low grade myoepithelial carcinoma measuring 15x8x5mm but with a positive margin at the deep peripheral margin at 7 o’clock over an approximate distance of 10mm.
The consequences of a more radical excision had concerning implications for the patient’s occupation as a hair dresser, and so the decision was made to opt for a potentially function preserving re-excision with post operative brachytherapy for recurrence prevention. A third resection was performed 2months after the second resection, with the surgical cavity re-excised including the superficial component of BR and extra resection performed at the site of positive margin. 10 brachytherapy rods were inserted transversely laid on the surface of the muscle along the 12cm wound. Histopathology revealed scar tissue with skeletal muscle and fat with no residual tumour identified in the specimen and the patient was declared to have had a margin clear resection. Brachytherapy was performed for one week and the rods removed.
Post operatively the patient experienced some discomfort in the right arm, with some limitation of strength and dysaesthesia along the scar. At 6month follow up the patient is clinically well.
Myoepithelial (ME) tumours primarily arising in soft tissue are rare,2 and recent studies have shown that there is a wide morphological and immunophenotypic spectrum of soft tissue ME tumours which show similarity to their salivary gland counterparts, with both benign and malignant subtypes.2,3 This variability often poses challenges in diagnosis, and a broad differential diagnosis may need to be considered in dealing with these tumours.4 They have been included by the WHO classification of soft tissue tumours in a continuum which includes myoepithelioma, mixed tumour and parachordoma.1 Previously, salivary gland ME tumours have been relatively well described inthe literature with a spectrum encompassing mixed tumours, myoepithelioma and myoepithelial carcinoma,5 however, ME tumours have been described recently in other parts of the body including upper and lower limb, breast, lung, sinonasal cavity, palate, retroperitoneum, vulva, testis and bone.6
Recently, cutaneous ME tumours have also been described, including mixed tumours of the skin (chondroid syringoma) and cutaneous myoepithelioma.4,7
Presentation and epidemiology
The largest series to date by Hornick et al.,6 presents 101patients with ME tumour of soft tissue. In this series, 41 tumours were located in the lower limb/limb girdle with 8 of these in the groin and 5in the buttock; 35 tumours were located in the upper limb/limb girdle with 7 of these located in the forearm. A broad age distribution between 3 to 83years with a peak incidence within third to fifth decades has been reported, and if there is any preponderance between sexes it is minimal.6 The most common clinical presentation is a mass with or without pain, but patients can also present with pain unrelated to a mass, swelling, paraesthesia and also as an incidental finding on examination.
Histopathological findings and criteria for malignancy
Due to the plasticity of myoepithelial cells, there is a wide spectrum of architectural patterns and cell types. Typically, soft tissue ME tumours are well circumscribed, may have a multinodular appearance,4 and are composed of epithelioid cells arranged in cords and sheets in a myxoidstroma.2,4 Necrosis is reportedly rare, however extensive necrosis was seen in case 1 of our mini-series. The wide variation in histopathological appearance means a long list of differential diagnoses often need to be considered, the main ones being extraskeletal myxoid chondrosarcoma, ossifying fibromyxoid tumour and epithelioid schwannoma.2
Immunohistochemical (IHC) studies are useful, with EMA and S-100P being good initial tests. If diagnosis is still in question, broad spectrum cytokeratins such as AE1/AE3, p63, GFAP, calponin, CK5/6, SMA can also be helpful. One series of 14 cases of ME tumours found 93% of the tumours had positivity for at least one of the epithelial markers, and found that 83% were positive for EMA, 85% were positive for S-100P and 50% were positive for GFAP.2 In addition, staining for INI-1 may be useful; it is a marker for loss of expression of this gene on the long arm of chromosome 22 and it is negative in at least 50% of ME tumours.8
There have been recent studies conducted into using genetics to aid in ME tumour diagnosis, particularly in uncertain cases. In particular, the EWSR1 gene rearrangement (Ewing sarcoma breakpoint region) has been shown to be relatively frequent in ME tumours of deep-seated soft tissue and ME tumours of bone, with 30 out of a series of 66 ME tumours showing the presence ofEWSR1 gene rearrangement in one reported series. Interestingly, it was found that ME tumours negative for EWSR1 gene rearrangement were more often benign, cutaneous or superficially located in soft tissues.9
Unlike in salivary gland ME tumours, where an infiltrative growth pattern is used as a predictor of malignancy, infiltrative margins are present in nearly half of ME tumours of soft tissue and its presence does not correlate with the clinical behaviour of the tumour.4 The degree of cytologicalatypia is important and is the only parameter which has been demonstrated to be predictive of higher recurrence and metastastic rates; tumours which show moderate or severe cytological atypia (prominent nucleoli, vesicular or coarse chromatin, pleomorphism) should therefore be classified as ME carcinoma (high grade).6 Those with benign or mild cytological atypia should be classified as myoepithelioma (low grade).4,6
Management and considerations in treatment
The mainstay of therapy for ME tumours is complete surgical excision with clear margins, given that both benign and malignant ME tumours of soft tissue have the potential for local recurrence, with malignant tumours having a higher risk.4 A recurrence rate of 18% versus 42% for benign ME tumour versus malignant ME tumour respectively was demonstrated in the series by Hornick et al.,6 at follow up. Hornick et al.,6 found that no clinical or pathological features apart from cytologicatypia (including age, status of excision margins, tumour depth, size, infiltration, necrosis, and mitotic rate) have been found to clearly correlate with local recurrence rate. However, Gleason et al.,10 found that the margin status was predictive of risk of local recurrence in their study of 29paediatric ME tumours, with 8 out of 10patients experiencing recurrence with marginal excision versus only 1 out of 6 patients experiencing recurrence when wide excision was performed.
Metastatic spread of ME tumours of soft tissues is well documented. Low grade ME tumours are unlikely to metastasise but there is at least 1 case report of a benign cytology ME tumour with regional lymph node metastasis.7 Rates of metastasis for high grade ME tumours up to 32% in adults6 and up to 52% in children10 have been reported suggesting possibly more aggressive disease in children. Metastases occur in lymph nodes, visceral organs and bone.11
It is not clear whether there is a role for adjuvant radiotherapy or chemotherapy. One case report has detailed success with carboplatin and paclitaxel in treatment of high grade metastatic ME tumour of the vulva, with a 3year remission achieved.12 Additionally, Gleason et al.10 found that 1 out of 7 patients who underwent chemotherapy for distant metastatic disease had a successful clinical response to doxorubicin and irosfamide with complete radiological resolution of pulmonary deposits. In Case 1 outlined above neoadjuvant chemoradiotherapy resulted in minimal reduction of tumour size preoperatively and in case 2, our patient underwent adjuvant brachytherapy with her third resection to reduce risk of local recurrence.
ME tumours are rare but as awareness increases they are probably becoming more frequently identified. Case 1 is unusual in that the patient presented with three separate deposits of ME tumour; it is possible that the two smaller deposits represented locoregional metastasis however it is interesting that all 15 regional lymph nodes examined were negative. Case 2 highlights the difficulty in individualising the treatment approach to the patient to balance morbidity with utilising wide local excision to achieve negative margins. Triple excision with adjuvant brachytherapy in the end resulted in a successful outcome, and to date both patients are clinically well. Further study into adjuvant radiochemotherapy is required to establish their role in treatment of ME tumours.
None
The author declares no conflict of interest.

©2015 Ting, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.