MOJ
eISSN: 2381-179X


Case Report Volume 11 Issue 4
1Department of Nephrology Penn State College of Medicine, USA
2Department of Cardiology, Penn State College of Medicine, USA
Correspondence: Muhammad Abdulbasit, Department of Medicine, Nephrology Division, Penn State College of Medicine, USA
Received: May 18, 2021 | Published: August 10, 2021
Citation: Abdulbasit M, Nudy M, Pfeiffer M, et al. Left ventricular outflow tract obstruction after transcatheter mitral valve replacement: the importance of preprocedural planning and prevention. MOJ Clin Med Case Rep. 2021;11(4):116-119. DOI: 10.15406/mojcr.2021.11.00395
Given the prevalence of mitral valve pathology in high-risk patients, transcatheter mitral valve replacement (TMVR) is becoming an attractive treatment modality. A known complication of TMVR is left ventricular outflow tract obstruction (LVOT) due to the prosthetic mitral valve and native anterior mitral valve leaflet encroaching into the LVOT. This is a serious complication which can lead to decreased cardiac output and death. Preprocedural planning with various imaging modalities (multi-detector cardiac CT and echocardiography) can predict those at high risk of LVOT obstruct. To increase awareness and to prevent this complication from occurring in the future, we present a case of LVOT obstruction after TMVR.
Highlights
Keywords: transcatheter mitral valve replacement, left ventricular outflow tract obstruction, multi-detector ct scan, valve in ring
Mitral valve disease, in particular mitral valve regurgitation, is a very common valve abnormality.1 Many patients with mitral valve disease have co-morbidities and prior sternotomies which can make open mitral valve repair and replacement high risk procedure and ultimately prohibitive. Furthermore, treating patients with degenerative prosthetic mitral valves or failed annuloplasty rings can provide a unique challenge. A new and emerging treatment technique is transcatheter mitral valve replacement for these populations at high risk for operative intervention. However, given the mitral valve’s proximity to the LVOT (area between the anterior mitral valve leaflet and the interventricular septum2), LVOT obstruction after TMVR is an arduous complication that can result in morbidity and postprocedural mortality. Multi-detector cardiac CT scan along with modern software can reliably predict the post-TMVR LVOT (called neo-LVOT) with the ability to simulate neo-LVOT by placing an appropriately sized bioprosthesis in mitral valve position.
A Sixty-nine-year-old man with a past medical history of coronary artery disease and severe mitral regurgitation with mitral valve prolapse which was treated with coronary bypass grafts along with triangular resection of P2 and P3, closure of P1to P2 scallop and a 34mm Sorin 3D memo ring (CC distance 31.9mm, SL distance 26.1mm and area of 498mm2) annuloplasty. Eight months later, he developed a peri-prosthesis leak in mitral position along the interventricular septum side (9’o’clock position) with intravascular hemolysis which was closed with an Amplatzer DuctOccluder II (PDA2).
Six months later, the patient had continued shortness of breath and a transesophageal echocardiogram revealed severe mitral regurgitation with evidence of mild peri-prosthesis leak (video 2). Due to his elevated surgical risk (STS score of 9%),patient underwent a valve-in-ring (ViR) transcatheter mitral valve replacement (TMVR) with a 29mm Edwards Sapien3 via a trans-septal approach (using 14mm balloon for septostomy). Pre-procedure planning was done based on transthoracic & transesophageal echocardiography and multi-detector CT (without measurement of neo-LVOT with a simulated valve) showing long anterior leaflet (27mm), thick interventricular septum (1.5cm), LVOT diameter (1.8cm), aorto-mitral angle of 64 degrees (Figure 1, video 1). Immediately post TMVR, patient did well with stable hemodynamic but over next 2-3 days systemic pressures started to drop along with decline in renal function was noted. Trans-thoracic echocardiogram showed systolic anterior motion (SAM) of the anterior mitral leaflet with elevated LVOT gradient of 88mmHg (baseline LVOT gradient of zero mmHg prior to TMVR). Furthermore, there was evidence of a continued iatrogenic atrial septal defect with continuous left to right shunt. Percutaneous left ventricular assist device (Impella CP) was placed for hemodynamic support for 24 hours with improvement in renal function. The patient underwent alcohol septal ablation of 1st large septal perforator (injected 1.5ml of pure alcohol) with drop of LVOT gradient from 80 mmHg to 20mmHg. Next further septal ablation was performed by injecting another 1 ml of pure alcohol into the basilar branch of 2nd septal perforator with final LVOT gradient of 10mmHg (video 3).The patient was able to be discharged and his renal function recovered, however, he still continued to have NYHA functional class III symptoms.
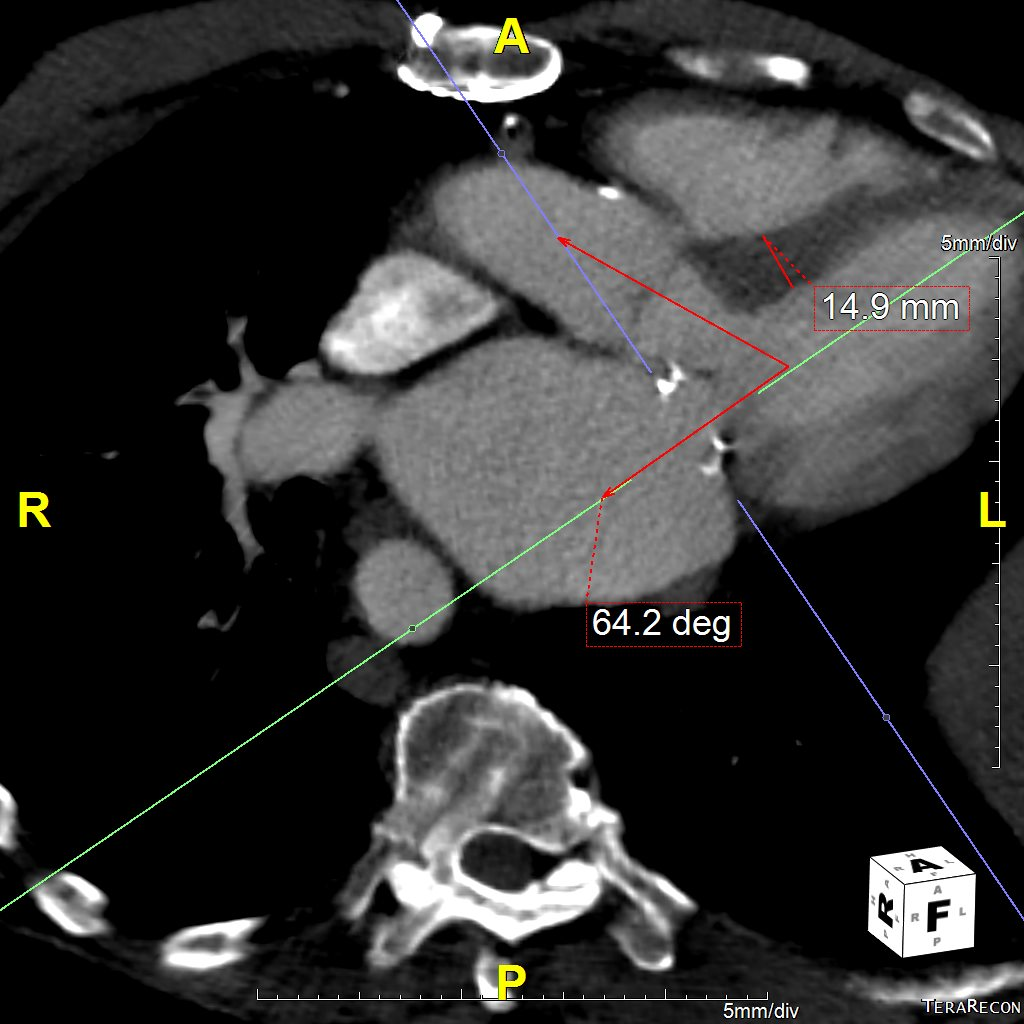
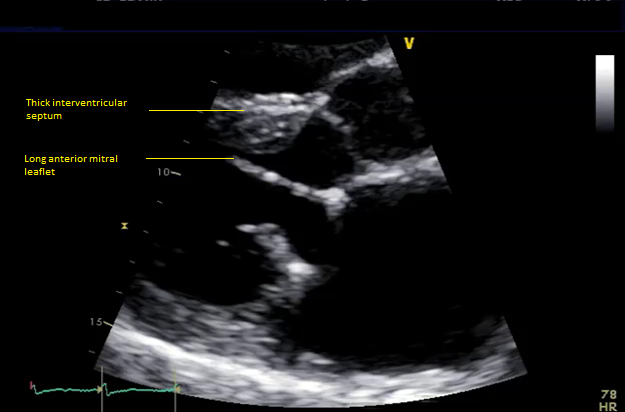
Figure 1 Pre-mitral valve replacement multi-detector CT scan showing thick interventricular septum and aorto-mitral angle (panel A), and transesophageal echocardiogram showing long anterior mitral leaflet and left ventricular outflow tract diameter.
Video 1 Transthoracic echocardiogram at baseline showing long anterior mitral leaflet and moderately thickened interventricular septum.
Video 2 Transesophageal echocardiogram showing severe mitral regurgitation with mild residual peri-valvular leak around Amplatzer duct occlude II.
Video 3 Successful alcohol septal ablation of medium sized septal perforator (panel A prior to ablation, panel B after ablation.
Due to ongoing clinical symptoms, he was then referred to our Interventional Cardiology team for a second opinion. A transthoracic echocardiogram on initial visit revealed severe LVOT obstruction which worsened with the Valsalva maneuver (peak velocity>4.5m/s) with a mean gradient across the mitral valve of 10mm Hg (Figure 2). Now post-TMVR, gatedchest computed tomography (CT) scan revealed evidence of the prosthetic mitral valve encroaching on the LVOT with small neo-LVOT, systolic anterior motion (SAM) of the native anterior mitral leaflet and persistent iatrogenic atrial septal defect with continuous left-to-right shunt (video 4). Left & right heart catheterization revealed neo-LVOT obstruction (baseline gradient 15-20mmHg) with a post-PVC gradient of 80mmHg and low cardiac index of 1.7L/min/m2. In addition, there was now an evidence of elevated gradient across mitral valve (mean of 10mmHg) on TEE as well as on cardiac catheterization assessment. Due to complex current clinical scenario including elevated trans-mitral gradients concerning for malfunctioning bioprosthesis, patient underwent a redo sternotomy with a 31mm St. Jude mechanical mitral valve replacement successfully. Intra-operatively, incomplete expansion of the Sapien 3 valve was noted causing altered leaflet mobility being the likely cause of elevated gradient across the bioprosthesis on pre-operative TEE. Post-op, the patient had a complete resolution of his symptoms.
With recent studies showing efficacy & safety of TMVR in degenerated bioprosthesis, failed annuloplasty rings, mitral annular calcification, and functional severe mitral regurgitation;3-6 transcatheter mitral valve replacement continues to be an emerging treatment for patients with symptomatic severe mitral valve disease at high or prohibitive risk for conventional mitral surgery. However, because of complex anatomy of the mitral valve apparatus, TMVR entails distinct and potentially fatal complications such as left ventricular outflow obstruction (LVOTO). It has been suggested that neo-LVOT obstruction occurs at a rate of 8.3% for ViR TMVR.7 For these reasons; proper pre-procedural planning to circumvent LVOT obstruction after TMVR is a must. It has been previously described that a smaller size of the natural LVOT, a smaller left ventricular size and a more acute angle between the aortic and mitral annular planes (referred to the aortomitral angle) are associated with an increased risk of LVOT obstruction after TMVR.8 Multi-detector row cardiac computed tomography (MDRCT) and transesophageal echocardiography have become the standard for pre-procedural TMVR planning.8 It is important to acquire images throughout the cardiac cycle as this allows for an assessment of the dynamic changes in the LVOT. It has been shown that end-systole is usually the time during the cardiac cycle when the LVOT-area is at its nadir.9
A recent multicenter, international registry among 194patients who underwent TMVR with a prior 3-deminsonal multidetector row cardiac CT scan, were analyzed for potential predictors of LVOT obstruction. Patients who underwent valve-in-valve (55.2%), ViR (25.8%), and valve-in-mitral annular calcification (19.1%) transcatheter procedures were included in this study. Those patients that developed LVOT obstruction after TMVR (13.6%) compared to those who did not, were at an increased risk of periprocedural death (34.6% versus 2.4%, p<0.001), conversion to surgical intervention (19.2% versus 1.8%, p=0.001), and emergent procedure percutaneously (38.5% versus 0.6%, p=0.001). Based on receiver-operating characteristic curve (ROC) analysis, an estimated neo-LVOT area of ≤1.7cm2 predicted LVOT obstruction with a sensitivity of 96.2% and a specificity of 92.3%.9 An additional multicenter TMVR registry which currently includes 38 patients (mean age 74.1 years) with a pre-procedural cardiac CT assessed patients for LVOT obstruction (defined as post-TMVR gradient difference greater than 10mmHg). Seven patients developed LVOT after the TMVR procedure. ROC analysis found a predicted neo-LVOT surface area of ≤189.4mm2 had 100% sensitivity and 96.8% specificity in predicting LVOT obstruction after TMVR.10 Small sample size in this study can lead to a wide confidence interval for the cut-off value used in this study.
There are various percutaneous interventional options have reportedly been used to prevent LVOTO prior to TMVR as well as treat it once has occurred post TMVR. Alcohol septal ablation has been previously described as a bail-out option for treating LVOT and was a failed technique used in the above patient case.11 Although use of this technique prior to TMVR may provide more gainful results by avoiding LVOTO and its’ related cascade of cardiogenic shock ultimately posing risk of bioprosthesis failure. However, this technique has an increased risk of conduction system abnormalities and an increased risk of pacemaker placement.12 Furthermore, percutaneous laceration of anterior mitral leaflet to prevent outflow obstruction (LAMPOON) prior to TMVR has recently been shown to be successful in five patients with prohibitive surgical risk and no other therapeutic alternatives (deemed not to be candidates for alcohol septal ablation) who were predicted to develop iatrogenic LVOT obstruction after TMVR based on echocardiography and MDRCT.13 Another study described a hybrid transatrial TMVR with transatrial resection of the anterior mitral leaflet prior to TMVR.14 LAMPOON has been prospectively investigated in a multicenter trial of 30 subjects at prohibitive risk of LVOT obstruction with 100% LAMPOON procedure success and 97% freedom from LVOT obstruction.15 Another option to treat this patient is to perform rescue LAMPOON to cut the protruding tip of the anterior mitral leaflet causing SAM after TMVR.16 This was not tried in this patient due to significantly elevated gradient across LVOT likely to be not responsive enough to rescue LAMPOON as well as due to elevated trans-mitral gradients concerning for bioprosthesis failure. In cases where LAMPOON is performed to gain larger neo-LVOT, skirt-LVOT should be measured on MDRCT using a simulated valve.
Also, some reports are available on few other interventional strategies that can be considered as a last resort in rightfully selected patient along with great deal of caution. The perfusion balloon (True TM Flow) has been used to be inflated in LVOT position as an opposing force during TMVR to avoid flaring of the valve struts toward LVOT.17 The same balloon inflation has been used in mitral valve position to assess LVOT obstruction with intra-procedural TEE prior to TMVR. Post TMVR, few interventional techniques such LVOT stenting or low TAVR has been used as last resort for LVOT modification in addition to alcohol septal ablation. On the same lines, high pressure balloon inflation in LVOT has been described to deform the mitral bioprosthetic valve strut away from LVOT in order to gain better neo-LVOT area.
We have presented a case of LVOT obstruction after TMVR which is not an uncommon complication (1 in every 7-8 TMVR overall; 1 in every other valve in MAC, 1 in every 12 valve in ring and 1 in every 50 valve in prior bioprosthetic valve).9 This is a complication that carries a high rate of morbidity and mortality (1 out of every 3 patient died).9 Given the increasing use of TMVR to treat high surgical risk patients, this is an important issue that needs careful attention. An in-depth knowledge and adroit use of available diagnostic modalities including MDRCT & TEE is of paramount importance to predict those patients at risk for LVOT obstruction in order to select appropriate treatment strategies to avoid unfavorable outcomes.
None.
The authors declare no conflicts of interest.
None.

©2021 Abdulbasit, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.