MOJ
eISSN: 2381-179X


Case Report Volume 2 Issue 4
Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Italy
Correspondence: Daniele Berardi, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Italy
Received: March 10, 2015 | Published: June 19, 2015
Citation: Berardi D, Badagliacca R, Poscia R, et al. Idiopathic pulmonary arterial hypertension: could we obtain a hemodynamic near normalization? Clues from three long-term survivors on epoprostenol treatment. MOJ Clin Med Case Rep. 2015;2(4):83-86. DOI: 10.15406/mojcr.2015.02.00027
Pulmonary arterial hypertension is a disease with a poor prognosis characterized by right ventricular failure, due to an increase in pulmonary vascular resistance and pulmonary arterial pressure. The introduction of specific treatments in the past decade has dramatically changed the management of the disease, and the addition of parenteral prostanoids to oral therapy has shown to improve survival in responding patients, as demonstrated by the clinical cases exposed in this paper: three female patients with moderate or severe pulmonary arterial hypertension and advanced NYHA functional class at diagnosis were able to overcome a 10-year survival along with a significant improvement in clinical and hemodynamic status thanks to the introduction of intravenous epoprostenol treatment.
Keywords: pulmonary arterial hypertension, epoprostenol, survival
CI, cardiac index; iNO, inhaled nitric oxide; iPAH, idiopathic pulmonary arterial hypertension; NYHA, new york heart association; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterization; RV, right ventricular; TAPSE, tricuspidal anular plane systolic excursion; WHO, world health organization; WP, wedge pressure; WU, wood units; 6MWD, 6-minute walking distance
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by an increase in pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP) leading to right ventricular failure and ultimately death.1 The past decade has witnessed major advances in PAH management, due to the introduction of specific therapies. Furthermore, oral drugs have been available in the last decade consenting a more wide treatment of the disease among clinicians’ community. ET (endothelin)-receptor antagonists and PDE-5 (phosphodiesterase-5) inhibitors showed to improve exercise capacity, NYHA functional class, hemodynamics and progression of disease in several clinical trials.2–6 However, many patients have clinical worsening during oral treatment. Thus, addition of prostanoids represents an important chance to reach clinical improvement for these patients. During a 16-year period of activity in our referral center we treated almost 500 severe PH patients, and near 100 patients were exposed to parenteral prostanoids. In this paper we describe the clinical course of 3 long-term survivor patients (>10years), as they could be considered to have had an exceptional good response to treatment.
Patient 1 is a 65-year-old woman with idiopathic pulmonary arterial hypertension (iPAH) diagnosed in 1997 after a rapidly progressive reduction of exercise capacity in the last year. At first evaluation at our center, the patient was in NYHA/WHO functional class III, with a reduced effort capacity (six-minute walk distance - 6MWD 370meters). ECG showed right ventricular (RV) strain, echocardiography showed marked RV dilatation, with a still preserved systolic function (tricuspidal anular plane systolic excursion - TAPSE 22mm). Right heart catheterization (RHC) revealed a moderate precapillary pulmonary hypertension (mean PAP 42mmHg, wedge pressure - WP 9mm Hg, cardiac index - CI 2.6l/min/m2, pulmonary vascular resistance - PVR 7.8WU–Wood Units), with no acute vasoreactivity to inhaled nitric oxide (iNO, 20ppm) (Figure 1). At that time, continuous iv infusion of epoprostenol was the only treatment available, so it was started and up-titrated to the maximum tolerated dosage. During the following months we observed a progressive improvement in clinical status and effort tolerance. After 2years (epoprostenol dose 32ng/kg/min) she was in class II with a good effort tolerance (6MWD 490m), and a second invasive evaluation documented an improvement of hemodynamics (mPAP 26mmHg, CI 2.9l/min/m2, PVR 4.7WU). In the following 15years, she improved gradually and currently she is on epoprostenol 59ng/Kg/min in NYHA/WHO class I with a good effort tolerance (6MWD above 500meters). ECG and echocardiography show right ventricular reverse remodelling, with normalization of RV dimensions and regression of RV strain signs. These results are consistent with the last RHC that shows a mild increase of PVR with normal pulmonary pressure (mPAP 22mmHg, CI 2.8l/min/m2, PVR 3.6WU) (Figure 1). During this period, the patient experienced only mild side effects due to prostanoids (flushing and tolerable jaw pain). We had to reposition the central venous catheter (CVC) twice due to local infection (6 and 10years after the first implantation).
Patient 2 is a 47-year-old woman who received a diagnosis of iPAH in 1999 after progressive dyspnoea and reduction of the exercise capacity in the last 2years and syncope in the last months. At first evaluation at our centre, the patient was in NYHA/WHO functional class III and had jugular distension, hepatomegaly and peripheral oedema. The 6MWD revealed a marked reduction of effort capacity (270m), ECG showed RV strain Figure 2A, echocardiography showed marked right heart dilatation with severe RV free wall hypokinesis (TAPSE 12mm) (Table 1). The RHC revealed a severe pulmonary hypertension (mPAP 57mmHg, WP 3mmHg, CI 2.2l/min/m2, PVR 16.4WU), with no response to the acute vasoreactivity test (iNO, 20ppm) (Figure 2). Thus, she was started on bosentan 62.5mg bid, increased to 125mg bid after 4weeks. In the first 10months the patient had an improvement in NYHA/WHO functional class (from III to II), in effort tolerance (from 270m at baseline to 360m after 3months of treatment) and a regression of systemic congestion. At that time RHC confirmed ahemodynamic improvement. During the following year, she had a new reduction of exercise capacity and returned to III NYHA/WHO functional class. A RHC, performed after 2years of treatment, showed a deterioration of pulmonary hemodynamics (mPAP 50mmHg, CI 2.4l/min/m2, PVR 12.8WU) (Figure 3).
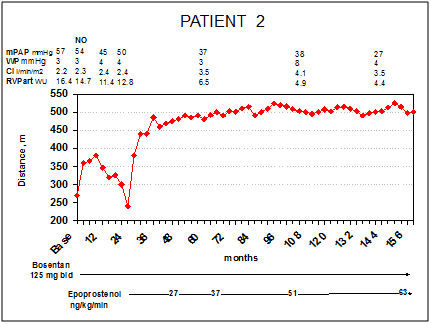
Figure 3PATIENT 2: Right heart catheterizations, 6MWD and treatment during follow-up. Legend – mPAP, mean pulmonary arterial pressure; WP, wedge pressure; CI, cardiac index; PVR art, arteriolar pulmonary vascular resistance, WU, wood units Presented CVC infection in her clinical history
On the basis of these results we started iv infusion of epoprostenol. The patient met a rapid improvement of NYHA/WHO functional class (from III to II after 2months of treatment) and effort tolerance (6MWD from 240m before epoprostenol to 380m after 2weeks, 445m after 4months, 485 after 1year and above 500m after 4years of treatment, (Figure 3)). Four years later (epoprostenol 37ng/Kg/min), RHC showed a considerable improvement of hemodynamic assessment (mPAP 37mmHg, CI 3.5 l/min/m2, PVR 6.5 WU), reversing PH to a mild stage. Thirteen years after starting epoprostenol, patient is still NYHA/WHO functional class II, 6MWD is stable on 500m; echocardiography (Table 1) and ECG Figure 2A-B show an improvement of RV eccentricity index and RV strain, respectively. The last catheterization shows a near normalization of pulmonary pressure and a mild elevation of PVR (mPAP 27mmHg, CI 3.5 l/min/m2, PVR 4.4WU) (Figure 3). Patient’s side effects during follow-up were essentially limited to flushing, jaw pain and episodic diarrhoea.
|
Baseline |
End follow-up |
EI Diastole |
1.75 |
1.25 |
EI Systole |
1.63 |
1.13 |
RA Area (Cm2) |
35 |
23 |
TAPSE (Mm) |
12 |
21 |
Tricuspid Regurgitation |
mild-moderate |
mild |
Pericardialeffusion |
none |
none |
Table 1 Patient 2: Echocardiographic parameters variations during follow-up
Legend – EI, eccentricity index; RA, right atrium; TAPSE, tricuspidal anular plane systolic excursion
Patient 3 is a 48-year-old woman who received a diagnosis of iPAH in 1999. Since 1998 she had dyspnoea and progressive reduction of effort tolerance, and these symptoms worsened during the last 2months of pregnancy. Just few days after the delivery she had an echocardiographic evaluation that showed an estimated systolic PAP of 100mmHg, so she was referred to our center. At first evaluation, the patient presented jugular distension (1-2/4), hepatomegaly and mild peripheral oedema; she was NYHA/WHO functional class III and showed a decreased exercise capacity (6MWD 330m, (Figure 4)); ECG showed right ventricular strain; respiratory function tests, perfusion lung scan and autoanticorpal assessment were normal. The patient underwent RHC that confirmed the diagnosis of moderate-severe precapillary PH with positive response to acute vasoreactivity test with iNO 20ppm (mPAP from 46 to 36mmHg, CI from 2.8 to 3.6 l/min/m2, PVR from 7.8 to 4.7WU) (Figure 4). According to these results, the patient was started on a calcium channel blocker (nifedipine 90mg/die; it was not possible to increase the dose because of severe peripheral oedema). After 1year of treatment, the patient showed a substantial increase in effort tolerance (6MWT from 330 to 470m, (Figure 4) and improvement of NYHA/WHO functional class (from III to II), but in the following 3months exercise capacity worsened, so oral beraprost was added at the dosage of 40μgqid and increased at the maximum tolerated dose (400μg/die). During the first year, the patient had a transient improvement in clinical status and exercise capacity, but during the following months she had a new severe deterioration, returning to NYHA functional class III. We performed a hemodynamic evaluation that revealed a significant worsening of pulmonary hypertension (mPAP 90mmHg, CI 2.8l/min/m2, PVR 14.4WU). Moreover, there was no more vasoreactivity. At that time we discussed the opportunity to start epoprostenol, but the patient refused the implantation of the intravenous line. For this reason we decided to suspend beraprost and nifedipine, and start bosentan (62.5mg bid for the first 4week, followed by 125 mg bid). After four months she had minimal clinical improvement and a RHC showed a decrease in PAPm, and PVR but also CI was reduced (mPAP 65mmHg, CI 2.2 l/min/m2, PVR 13 WU) (Figure 4). So epoprostenol was started and up-titrated to the maximum tolerated dosage (up to 46ng/kg/min during a 10years follow-up). During this period we obtained a progressive and sustained improvement of clinical status (WHO class II) and effort tolerance (6MWD above 500m). At the ECG we observed a reduction in RV strain and echocardiography documented a reverse remodelling of RV geometry (Figure 5). RHCs performed 2 and 4years after starting epoprostenol revealed a progressive improvement of pulmonary hemodynamics (mPAP from 65mmHg after bosentan treatment to 51 and 36mmHg, respectively); last evaluation confirmed the results obtained (Figure 4). Flushing and jaw pain were the most frequent side effects referred by the patient during follow-up. Liver function tests have been monthly performed, remaining between normal values. The patient never presented CVC infection in her clinical history.
PAH is a progressive disease, with a very poor prognosis if left untreated.7 In the last decade we witnessed the introduction of specific therapies which showed to improve exercise capacity, NYHA functional class, hemodynamics and progression of disease in several clinical trials.2–6,8–11 Despite the number of PAH patients on treatment is increasing worldwide, there are no data published on patients with a very long survival and a good hemodynamic response. In this paper we report three iPAH cases with a very long survival (>10years), impressive hemodynamic response (almost normalization of PVR) ad reverse remodelling of the RV. This response to specific treatment is uncommon and is similar to the results obtained in the minority of patients who could be treated with calcium channel blockers (CCB).12 Among our cases, only one patient was vasoreactive, but she had limited benefit by CCB; after few months she had a severe deterioration as she lost pulmonary vasoreactivity. Thus our patients had “fixed” PVR when epoprostenol was started, and the very significant reduction in PVR after long–term treatment raises the question if it is possible to achieve the regression of the pulmonary arteriopathy responsible of the clinical syndrome. Several studies demonstrated that prostanoids have antiproliferative effects in vitro13–16 and can prevent or reverse the pulmonary arteriopathy in animal models of pH.17 A similar mechanism could be present in human, as suggested by the fact that patients, who did not have acute hemodynamic benefit during acute vasodilator challenge, had clinical and hemodynamic benefit during chronic epoprostenol treatment.18 The results of our patients are even more impressive with the near normalization of PVR, RV anatomy and function. In our opinion, the magnitude of this improvement suggests that in a small subgroup of non-vasoreactive patients with iPAH it might be possible to obtain a reverse remodelling of the obstructive arteriopathy. This hypothesis should be confirmed by the analysis of large registries which are currently ongoing,19–21 and it should foster collaborative studies among referral centers looking at these patients with a particular good response to therapy.
None.
The author declares no conflict of interest.

©2015 Berardi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.