MOJ
eISSN: 2381-179X


Case Report Volume 11 Issue 3
1Department of Otorhinolaryngology, People’s Hospital of Ordos Dongsheng District, China
2Department of Neurology& Neurosurgery, People’s Hospital of Ordos Dongsheng District, China
3Department of hyperbaric oxygen therapy, The affiliated hospital of Inner Mongolia Medical University, China
4Beijing Ivheart Health Management Center, China
Correspondence: Chunhui Yang, Beijing Ivheart Health Management Center, China
Received: April 20, 2021 | Published: May 3, 2021
Citation: Han Y, Zhang J, Ning R, et al. Hyperbaric oxygen therapy caused tympanic membrane and ventricular hemorrhage: a case report. MOJ Clin Med Case Rep. 2021;11(3):60-62. DOI: 10.15406/mojcr.2021.11.00382
A 40-year-old male undergoing rehabilitation with hyperbaric oxygen therapy (HBOT) three months after an acute right frontal lobe cerebral infarction. On the second day of HBOT, he felt a significant blockage and pain in his left ear during and after the treatment. The endoscopic assessment of the ear and nose revealed haemorrhage in the left ear tympanic membrane and hemorrhagic effusion in the tympanic chamber. The nasal septum was found to be left deviated resulting in significant narrowing of the left nasal cavity and significant poor ventilation. Laboratory tests showed normal blood count and normal blood coagulation count. The final diagnosis of haemorrhage and fluid accumulation in the tympanic chamber of the left ear was made. After the treatment of the middle ear ball blowing once a day for 10 days, the pure tone audiometry of the left ear reached the level of the right ear, and the blockage and pain in the left ear disappeared completely. The orthoscopy inspection indicated total absorption of hemorrhagic fluid in the tympanic chamber of the left ear. This is the first report that HBOT caused tympanic membrane haemorrhage and offers new insights into the prevention of comorbidities in HBOT.
Keywords: hyperbaric oxygen therapy (HBOT), tympanic membrane haemorrhage, tympanic ventricular effusion, middle ear barotrauma(MEB)
Hyperbaric oxygen therapy (HBOT) as an important innovative therapy has been widely used in clinical practice for two decades and has achieved satisfactory effects,1-9 particularly in the areas of acute carbon monoxide poisoning,7 gas gangrene8 and decompression sickness.4
At present, HBOT was taken as one of the safest therapies,4,9 even so, there are side effects associated with it. Middle ear barotrauma (MEB) is one of the most common side effects seen in HBOT.1,5 The other potential risks include temporary eye lens changes,15-18 lung collapse,12,13,19,20 seizures,21,22 hypoglycemia in diabetes patients treated with insulin.10,11 Here we presented a case of HBOT caused tympanic membrane and ventricular haemorrhage for recovery of the brain infarction. As we known, this may be the first report for HBOT caused tympanic membrane haemorrhage. This study should lead to better consideration of risk and benefit in discussions with the patient when considering HBOT.
Recent studies identified that HBOT enhance cerebral circulation, reduce ischemia-reperfusion injury, and lessen the extent of irreversible neurological impairment.6 And even HBOT can activate the neuroplasticity of brain tissues in chronic stroke patients.24
The patient was 40-year-old male. He was hospitalized for acute infarction of the right frontal lobe and was transferred to our hospital for HBOT rehabilitation treatment after his condition stabilized. He had a 2-year history of hypertension and denied a history of diabetes mellitus. He had no medical conditions such as ear congestion or blocked ears, high myopia, or large pulmonary alveoli prior to HBOT. The patient also denied a history of chronic rhinitis. The patient had been orally taking a small dose of aspirin enteric tablets, 100mg/day for one year.
On the first day of HBOT, it took 20 minutes to pressurize the oxygen chamber from 1 to 1.4 atmospheres absolute (ATA) during which the patient experienced only a transient sensation of stuffiness in the left ear and no discomfort afterwards. Therefore, during the second HBOT, the rate of oxygen discharge from the chamber was adjusted from 1 to 1.6 ATA after 20 minutes, during which he felt a significant blockage in his left ear and pain at the same time. The discomfort did not go away after the treatment, so he came to the otology department for the diagnosis.
Otoscopic examination revealed hemorrhage in the left tympanic membrane and hemorrhagic effusion in the tympanic chamber (Figure 1A), while the right ear showed no significant abnormality. The nasal endoscope showed a moderate to severe leftward deviation of the nasal septum, squeezing the inferior turbinate, and a significant narrowing of the common nasal passage, with the 4 mm endoscope unable to fully penetrate (Figure 1B). The pharyngo-pharyngeal opening can be seen in the slit of the middle nasal passage, and the shape and activity of the pharyngo-pharyngeal opening can be seen when the patient made swallowing movements (Figure 2). While the right nasal cavity was spacious and well ventilated, and there was no obvious abnormality in the morphology of the pharyngeal orifice of the eustachian tube. CT scan of the ear ruled out cholesterol granuloma of the tympanic chamber and sinus. Laboratory tests showed normal blood count as well as normal coagulation function. Blood pressure was 140/104mmHg (1mmHg=0.133kPa) during HBOT.
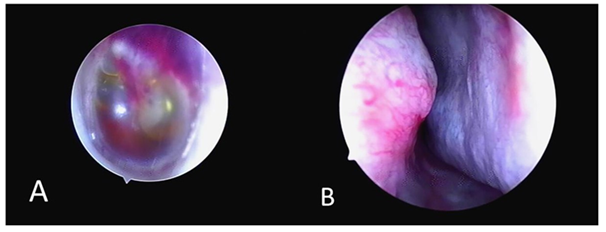
Figure 1 (A) Bleeding and fluid accumulation in the tympanic chamber of the left ear on endoscopic examination. (B) Nasal endoscopy revealing a moderate to severe curved left deviation of the nasal septum, compression of the inferior turbinates, and significant narrowing of the common nasal tract.
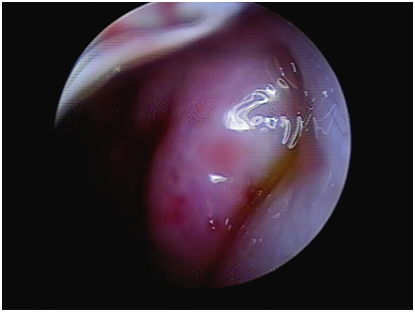
Figure 2 Nasal endoscopy showing the eustachian tube round pillow and pharyngeal opening in the left middle and posterior part of the middle nasal tract.
Due to the narrowing of the left nasal cavity, the blowing of the eustachian tube could not be performed directly, so the blowing of the middle ear bulb of the left eustachian tube was performed, and the patient immediately felt a significant reduction of the obstruction in the left ear. The middle ear ball blowing treatment was performed once a day, and after 10 days, the left tympanic chamber was found to have absorbed all the fluid and the pure tone audiometry of the left ear was equal to that of the right ear.
In a HBOT chamber, the air pressure is increased two to three times higher than normal air pressure.4 A common complication of HBOT is tympanic ventricular effusion,1,2,4 while tympanic ventricular hemorrhagic effusion has not yet been reported. HBOT causes tympanic effusion mainly because of the pressure imbalance between the inside and outside of the tympanic chamber.2 The negative pressure in the tympanic chamber leads to the leakage of blood vessels and mucosal interstitial fluid into the tympanic chamber, resulting in tympanic effusion. However, tympanic ventricular hemorrhage does not usually occur. Fijen et al. studied 34 patients on antiplatelet/anticoagulant medication who received HBOT compared to 39 controls who were not taking any medication and showed that no tympanic bullae hemorrhage was found in the experimental group, suggesting that HBOT does not usually cause tympanic bullae hemorrhage.25 The tympanic bullae hemorrhage in our case may be the result of a combination of multifactor, including1 poor ventilation of the tympanic chamber of the left eustachian tube due to significant arcuate left deviation of the nasal septum, leading to increased brittleness and permeability of the mucosal vessels in the tympanic chamber and tympanic membrane;2 the patient's long-term aspirin use has diminished platelet aggregation in the blood;3 the patient had a history of hypertension for two years and his blood pressure was 140/104mmHg during hyperbaric oxygen therapy;4 the increase in 1.6 ATA during the second hyperbaric treatment, although within the conventional safety range, may have been too high a relative pressure for this patient.
It was well known that the tympanic membrane and tympanic chamber are important structures for sound formation and conduction.2 If the tympanic membrane and chamber become diseased, conductive hearing loss with ear congestion, tinnitus, ear blockage and other discomfort will occur.1,2,3,5 An important structure that can maintain the proper physiological function of the tympanic membrane and ventricles is the eustachian tube.3 Under normal conditions, the normal function of the eustachian tube is maintained mainly by the normal contraction and diastole of the palatine sail tensor, palatine sail raphe and supraglottis muscles.3 Therefore, if the fresh ventilation of the nasopharynx is not sufficient when swallowing, it will make the quality and quantity of gas entering the tympanic chamber does not meet the demand of the chamber to maintain normal air pressure and oxygen, and it will make the mucosa and blood vessels inside the chamber become fragile and damaged.3
In summary, our study suggested thoroughly understanding of the potential side effects of HBOT is critical to provide safe medical care with complete patient informed consent. According to this report, we recommend that rhinoscopy should be done routinely before HBOT to detect conditions such as nasal septal curvature or nasal obstruction. For patients with deviated septum, if feeling ipsilateral ear stuffiness and swelling during HBOT, measures must be taken to prevent the formation of excessive negative pressure in the tympanic chamber, with the aim of maintaining the relative balance of air pressure inside and outside the tympanic chamber and preventing the occurrence of tympanic hemorrhage and hemorrhagic effusion. The measures such as intermittent swallowing or giving ephedrine nasal drops before undergoing HBOT will be helpful. This case report provided an extended information for providers to identify, understand, and quantify these side effects in clinical practice.
The author declares no conflict of interest.
None.
None.

©2021 Han, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.