MOJ
eISSN: 2381-179X


Case Report Volume 13 Issue 2
1Neurosurgery Department, University Hospital Center of Guadeloupe, Guadeloupe
2Abdou Moumouni University of Niamey, Department of Neurosurgery, National Hospital Center of Niamey, Niger
3Research Department of Sub-Saharan Africa Futures Neurosurgeons Association (SAFNA), Cotonou, Benin Republic
Correspondence: Dossou Mèhomè Wilfried, Neurosurgery Department, University Hospital Center of Guadeloupe, Guadeloupe
Received: April 24, 2023 | Published: May 15, 2023
Citation: Dossou MW, Al-Hassana I, Tankari A, et al. Esthesioneuroblastoma diagnostic and therapeutic difficulties: a case report from the University Hospital of Guadeloupe. MOJ Clin Med Case Rep. 2023;13(2):33-36. DOI: 10.15406/mojcr.2023.13.00432
Background: An esthesioneuroblastoma or olfactory neuroblastoma is a rare nasosinusal tract tumor developed at the expense of the olfactory neuroepithelium, the first case of which was described in the literature in 1924 by Berger et al. The management of this tumor is reserved for experienced surgical teams and no recommendations are available, the experience of each center being limited due to the rarity of this tumor. In this article, we report on our experience in one case.
Case report: A 65-year-old patient was brought to the emergency room for a seizure with post-critical coma in whom the history revealed a frontal syndrome associated with helmet headaches and hallucinations. Imaging allowed us to make the diagnosis of esthesioneuroblastoma. He was treated surgically by a mixed neurosurgery-otorhinolaryngology team. The removal was complete and followed by radiochemotherapy according to the STUPP protocol. Anatomopathology concluded the diagnosis of high-grade III and IV olfactory neuroblastoma. The clinical evolution of the patient postoperatively is favorable although vertigo persists.
Conclusion: An esthesioneuroblastoma is a rare tumor with mostly poor symptomatology even in cases of extensive intracranial invasion. Treatment is based essentially on the most complete surgery possible followed by radio-chemotherapy.
Keywords: esthesioneuroblastoma, diagnosis, Guadeloupe
An esthesioneuroblastoma or olfactory neuroblastoma is a rare tumor of the nasosinusal tract, developed at the expense of the olfactory neuroepithelium, the first case of which was described in the literature in 1924 by Berger et al.1 It accounts for 3 to 6% of sinus tumors but its exact incidence is difficult to establish because of the complex logical diagnosis of certainty.2 More recently, nuclear imaging, with (18F)-fluorodeoxyglucose (FDG) PET scanning, has also demonstrated its value, mainly in the diagnosis of distant extensions of recurrences.3 Approximately 1000 cases of esthesioneuroblastoma have been published in the literature, the majority of them in the last two decades, but no standard management has been validated to date. The management of this tumor is reserved for experienced surgical teams and no recommendations are available, as the experience of each center is limited due to the rarity of this tumor. In this article, we report our experience of a case by comparing the clinical, paraclinical, histological, and therapeutic data of this patient with those published in the literature.
A 65-year-old patient, known for recurrent episodes of vertigo treated with tanganil, was brought to the emergency department for a convulsive seizure associated with altered consciousness. He had been presenting for 6 months with temporospatial disorientation accompanied by incoherent speech, irritability, and auditory hallucinations.
The patient complained of unusual headaches, neck pain, incontinence, and morning epistaxis for several days.
Neurological examination reported a patient with normal alertness but confusion and agitated, discrete cervical stiffness without Brudzinski or Kerning signs.
A Cerebral CT scan shows filling of the right ethmoidal cells with lysis of the middle and lower turbinates associated with complete filling of the right frontal sinus and ethmoidal enhancement. There is a heterogeneous mass strongly enhanced by the right frontal contrast medium surrounded by a halo of edema creating a mass syndrome on the medial structures with infiltration of the white matter and subfascial involvement. This image would suggest an abscess with a sinus origin, a glioma cannot be ruled out (Figure 1).
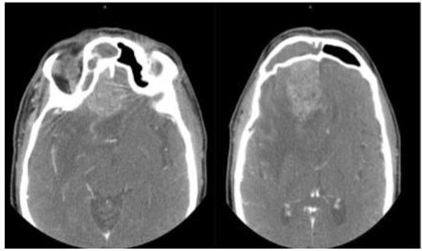
Figure 1 Cerebral CT scan with contrast injection showing the tumor associated with a large edema and midline deviation.
Magnetic resonance imaging (MRI) allowed us to make the diagnosis of an esthesioneuroblastoma (Figure 2).
Nasofibroscopy revealed an irregular framboise lesion in the right olfactory cleft, descending from the ethmoid and extending posteriorly without reaching the sphene-ethmoidal recess.
Endoscopy of the nasal cavities with a 30° optic reveals a framboise lesion occupying the right middle meat pushing back the middle concha, the cavum is empty, without any involvement of the sphene-ethmoidal recess.
The left nasal cavity is free of suspicious lesions but shows a left sphenoidal discharge.
He underwent a tumor removal by a mixed Otolaryngology-Neurosurgery team with a total removal of the tumor by endoscopic endonasal approach. Post-operative imaging showed complete removal of the tumor.
The postoperative period was marked by phantosmia, which regressed with a return of taste and smell. The examination revealed a patient oriented in time and space and the disappearance of hallucinations. Dizziness nevertheless persisted.
The anatomopathological examination concluded to a high-grade neuroendocrine tumor difficult to classify between a high-grade neuroblastoma and a high-grade neuroendocrine carcinoma but whose aspects sometimes typical of high-grade olfactory neuroblastoma made the review group conclude the diagnosis of high-grade olfactory neuroblastoma III and IV. Oncological management consisting of radiotherapy followed by chemotherapy was indicated for this patient.
An esthesioneuroblastoma originates from basal stem cells of the olfactory neuroepithelium. The tumor is phenotypically intermediate between a pure neural tumor (neuroblastoma) and neuroendocrine epithelial neoplasia (small cell carcinoma), and there are case reports of a vasopressin-secreting tumor.
The most common presenting symptoms are unilateral nasal obstruction and epistaxis; however, less common presenting symptoms include headache, facial pain, rhinorrhea, anosmia, diplopia, decreased visual acuity, exophthalmos, epiphora, and syndrome of inappropriate antidiuretic hormone secretion or a combination of these symptoms.4
Lapierre et al. in South Lyon found nasal obstruction (50%), headache (40%), and epistaxis (30%) as revealing symptoms in the majority of cases.5 Hachicha et al. in Tunisia reported a case with atypical symptomatology. Indeed, the patient was received for intracranial hypertension syndrome with the notion of a generalized tonic-clonic convulsive seizure.6 This case reports and series show that although the clinical presentation of an esthesioneuroblastoma is dominated by otorhinolaryngological signs, these signs are nonetheless specific.
In our case, the clinical presentation was dominated by confusion and temporospatial disorientation associated with hallucinations.
This tumor has been reported in a wide age range (2 to 94 years) and has no gender predilection, although studies have shown a male predominance.7 Some case series document a bimodal age peak in the second and sixth decades; however, others indicate a unimodal presentation in the fifth to sixth decades. The mean age in the series of Modesto et al. were 51 years with extremes of 8 to 80 years.8
In the series by A. Lapierre et al., seven men (70%) and three women (30%) were involved, with a mean age of 56 years at diagnosis and extremes of 32 and 83 years. Our case is comparable to the literature. Indeed, our patient is 65 years old. No environmental, geographic, or lifestyle risk factors were associated with an increased risk of esthesioneuroblastoma.
We did not identify any risk or associated factors in our patient, nor the literature of reported cases and series.
The stage of the disease at initial presentation is highly predictive of survival, and accurate staging is essential. Several classification systems have been proposed, including the modified Kadish, Hyams, and TNM classifications. The use of these proposed staging systems remains a matter of preference, as none is universally accepted.
The Kadish system (Kadish et al in 1976) is the most commonly used approach to classify the anatomical extent of an esthesioneuroblastoma.9 Kadish staging uses three categories, groups A, B, and C. The modified Kadish staging system includes a fourth step for patients with lymph nodes or distant metastases. An additional group D was proposed by Chao et al.10
Group A:
Group B:
Group C:
Group D:
Orbital invasion can also be classified according to the Lannetti classification11
Grade I:
Grade II:
This system has been criticized because it requires surgical staging, does not systematically take into account metastatic spread, and lacks prognostic value. Furthermore, few patients at initial clinical discovery have group a disease if staging is strictly applied due to a high incidence of ethmoidal sinus involvement. Simon et al. found no patients in their 20-year experience with stage A disease.12
In our case, the patient was classified as Kadish grade C as in the series of Modesto et al. who found a majority of Kadish group C patients (37.2%) and only 11.6% of group A patients.8 The series by A. Lapierre et al. reported a majority of Kadish group C cases (90%)(5). Similar observations have been reported in other series, and the overall incidence of Kadish A disease is estimated at 5%.13
The Dulguerov staging system uses the TNM classification and includes imaging data.9 Some authors prefer the Dulguerov system because early involvement of the cribriform plate is recognized at the T2 stage and intracranial but extradural tumors are separated from those with true brain involvement. According to Dulguerov's classification, our patient is admitted at stage T4N0M0 of his disease. This classification was not taken into account in the series of Modesto et al.,8 and Lapierre et al.5
Local invasion of an esthesioneuroblastoma occurs most commonly in the paranasal sinuses, orbits, and anterior cranial fossa. The metastatic disease most commonly involves local lymph nodes, with distant metastases to the lungs, liver, and bone.14 The incidence of cervical lymph node metastases ranges from 20-30% and reaches 44% in stage C.15
Howell et al. described a predictable pattern of cervical lymph node metastases, usually involving level II nodes (93%), with frequent involvement of level I (57%), level III (50%), and retropharyngeal nodes (43%).16 Modesto et al.,8 noted approximately 21% cervical lymph node extension, unlike Lapierre et al.,5 who did not note lymph node extension. In our case, there was no locoregional cervical lymph node extension.
The tumor is often very vascular and prone to heavy bleeding at biopsy. In our case, the biopsy found a framboise lesion occupying the right middle meat pushing back the middle horn without involving the sphene-ethmoidal recess. There was no active bleeding at the time of the operation and the end of the biopsy. Imaging, including computed tomography (CT) and magnetic resonance imaging (MRI), plays a key role in the diagnosis of esthesioneuroblastoma. These imaging modalities should always be included to properly assess the extent of the disease.
CT should be performed with thin sections (1 mm thick) and reformatted in both the coronal and sagittal planes. An esthesioneuroblastoma has no specific CT characteristics. On imaging, an esthesioneuroblastoma most often appears as a non-specific mass in the upper nasal cavity. However, CT is essential to assess the bony involvement of the sieve plate, ethmoidal fovea, and lamina papyracea. The classic appearance is that of a bilobed mass extending through the cribriform plate. Intratumoral mineralization may be a diagnostic clue for an esthesioneuroblastoma; however, this finding is also frequently seen in inverted papilloma and chondrosarcoma. The presence of cysts at the margins of intracranial tumor components has been described as diagnostic of aesthesioneuroblastoma, but this is rarely seen. The development of marginal cysts associated with dural metastases of aesthesioneuroblastoma may also be observed. Non-destructive bone remodeling is not uncommon, due to the indolent growth pattern in some cases. The mass shows moderate and uniform enhancement. Scattered mottled calcifications are sometimes present.17 CT is also useful for assessing locoregional extension and distant metastases.16
In our case, the brain scan was atypical. It showed the filling of the right ethmoidal cells with lysis of the middle and lower turbinates associated with complete filling of the right frontal sinus and ethmoidal enhancement. There was a heterogeneous mass strongly enhanced by contrast medium in the right frontal area surrounded by a halo of edema creating a mass syndrome on the medial structures with infiltration of the white matter and subfascial involvement. This image raised the suspicion of a sinus abscess without ruling out a glioma (Figure 1).
Magnetic resonance imaging allowed us to make a diagnosis of esthesioneuroblastoma. It rules out the nasosinusal abscess and strongly suggests a nasosinusal tumor.
Surgery remains the first-line treatment for these tumors and has been shown in meta-analyses to be an independent factor in progression-free survival and overall survival.18 Current treatment guidelines recommend complete surgical resection and adjuvant radiotherapy based on improved overall survival with this regimen. The surgical technique depends on the initial tumor stage of the disease. Historically, the operation of choice has been craniofacial resection, involving a bifrontal craniotomy to remove en bloc the cribriform plate, crista galli, adjacent dura mater, and olfactory bulbs and tract; this remains the standard operation in patients with locally extensive disease. For localized tumors, the trans-facial approach is currently preferred.19 However, the development of endoscopic surgery over the last decade has resulted in comparable carcinological outcomes, with limited surgical morbidity and length of hospital stay, particularly for stage T1 or T2 tumors.20 In meta-analyses, endoscopic resection seems to have better overall survival results than trans-facial surgery. These results should be considered with caution as the studies using trans-facial surgery are significantly older than those on endoscopic surgery. This fact may partly explain the more modest results.18
In the case of locally advanced tumors (invasion of the orbit or anterior brain fossa), a mixed surgical approach, either trans-facial or endoscopic and neurosurgical, is preferable. In our case, a mixed neurosurgical and endoscopic endonasal approach was preferred allowing complete resection of the tumor. This mixed approach was also found in 33% of the series by Lapierre et al. Despite the lack of studies with a satisfactory level of evidence, adjuvant radiotherapy is currently considered the standard treatment after surgery, whether histologically complete or not. Several retrospective studies have shown a benefit in terms of local control with the addition of adjuvant radiotherapy, sometimes with major differences in local recurrence-free survival between treatment with surgery or radiation alone and a combined approach (0%, 51%, or 87% respectively [p=0.03] in the study by Chao et al.10 However, no long-term overall survival benefit of adjuvant radiotherapy has been demonstrated.21
Exclusive radiotherapy should not be used unless surgery is contraindicated, because of significantly lower local control and specific survival rates compared to the combined approach. For example, analysis of the Surveillance, Epidemiology and End Results (SEER) database by Jethanamest et al. found a mean specific survival time of 92.8 months for patients treated with exclusive irradiation compared with 216.8 months for joint treatment (p < 0.02).22
The modalities of external radiotherapy are not clearly defined. In the Lapierre et al. series, the majority of patients were treated with the conformal technique, due to the year of management (before 2005), but intensity-modulated conformal radiotherapy (IMRT) should now be recommended, for sinus tumors, to limit acute and late toxicity associated with radiotherapy.23 Treatment volumes should contain at least the tumor bed and initial extensions as well as the initially invaded lymph nodes if present.24 Lymph node involvement, initially considered rare in this disease, at around 10%,25 appears to be more frequent in recent series, probably due to improved imaging techniques, with initial lymph node involvement rates of up to 50% for advanced tumors.26
Our patient underwent conventional radiotherapy combined with chemotherapy following the STUPP protocol.
With optimal management, the prognosis of an olfactory esthesioneuroblastoma is good, with median progression-free survival of 6.9 years and overall survival of more than 10 years, all stages combined.27 In this context, the management of acute toxicity, and especially late toxicity, is a major issue.
In the Lapierre et al. series, two patients (20%) suffered severe complications of the surgery, requiring hospitalization or even further surgery: a frontal abscess and an osteomeningeal breach.5 The development of less invasive surgical techniques and better intraoperative tracking (neuronavigation) should eventually reduce these complications.
Our patient had no early or short-term postoperative complications. The control imaging was satisfactory with complete resection. The role of systemic chemotherapy in the treatment of esthesioneuroblastoma is not yet well defined. Neoadjuvant chemotherapy was given to 24 patients, with a 71% response in the series by Modesto et al.8
An esthesioneuroblastoma is a rare tumor whose symptomatology is usually poor even in cases of significant intracranial invasion. Intracranial hypertension or epilepsy is exceptional (in our case). Treatment is based essentially on the complete surgery possible followed by radio-chemotherapy. Experts must reach precise recommendations in the management of this disease.
The patient's consent was obtained to publish this case as well as that of the head of neurosurgery.
The authors report no conflicts of interest.

©2023 Dossou, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.