MOJ
eISSN: 2381-179X


Case Report Volume 10 Issue 4
1Gastroenterology Department, Mohammed VI University Hospital, Morocco
2Physiology Department, Faculty of Medicine and Pharmacy at Cadi Ayyad University, Morocco
Correspondence: Yassine Lemfadli, Gastroenterology department, Mohammed VI University Hospital, Morocco
Received: July 01, 2020 | Published: July 13, 2020
Citation: Lemfadli Y, Bouchrit S, Lairani F, et al. Diffuse oligosymptomatic caroli’s disease: case report. MOJ Clin Med Case Rep . 2020;10(4):81-83. DOI: 10.15406/mojcr.2020.10.00349
This article describes a case of Caroli's disease in a 53-year-old female patient who complained non-specific abdominal pain without cholestasis or cholangitis. Ultrasound and hepatic magnetic resonance imaging showed segmental saccular dilations connected to intrahepatic bile ducts without hepatic fibrosis. This clinical case shows the possibility of having oligosymptomatic forms in the diffuse forms of Caroli disease, therefore the interest to consider this diagnosis in case of non-specific abdominal signs and to request a hepatic ultrasound.
Keywords: caroli disease, caroli syndrome, congenital bile duct dilatation
Caroli's disease (CD) is a rare congenital disease, first described in 1958 by Jacques Caroli.1 It is part of the spectrum of fibropolycystic liver pathologies. It is characterized by congenital non-obstructive dilation of the intrahepatic bile ducts. It can be localized or diffuse. Caroli syndrome is defined as its association with congenital hepatic fibrosis.2 It is most often revealed by recurrent episodes of cholangitis.3
This article describes the case of CD in a 53-year-old female patient with cystic formations distributed throughout the hepatic parenchyma, fortuitously diagnosed in adulthood, during the etiological investigation of non-specific abdominal pain.
A 53-year-old femal patient was seen in consultation. She complained of abdominal pain, which had started a year ago. The intensity of the pain was mild to moderate, intermittent, recurrent, located in the upper right quadrant and relieved by taking first-level pain relievers. The patient also reported urinary burns, but no fever or chills. She did not report jaundice, pruritus, vomiting, or weight loss.
The patient had no history of personal or family hepatopathy. She was taking no treatment and no toxic habits.
The clinical examination, in particular the abdominal, respiratory and cardiovascular examination, did not show any abnormalities. There was no jaundice or fever. The abdominal examination did not show hepatomegaly or signs of hepatocellular insufficiency or portal hypertension.
Hematological and biochemical examinations were normal (Table 1). There was no cholestasis or cytolysis. The levels of alpha-foeto-protein and carcinoembryonic antigen were normal. The cytobacteriological examination of the urine was sterile.
Parameters |
Results |
Hemoglobin |
12.3 (g/dL) |
Hematocrit |
37,3 (%) |
Leukocytes |
4950 (/mm3) |
Platelets |
191 (/mm3) |
Prothrombin activity |
100 (%) |
C-reactive protein |
1,6 (mg/L) |
AST |
21 (UI/L) |
ALT |
12 (UI/L) |
GGT |
21 (U/L) |
Alkaline phosphatase |
67 (U/L) |
Total bilirubin |
6 (mg/L) |
Albumin |
46.8 (g/L) |
α-Fetoprotein |
1.6 (ng/mL) |
CEA |
3 (ng/mL) |
LDH |
349 (U/L) |
Urea |
0.35 (g/L) |
Creatinine |
6 (mg/L) |
Table 1 AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; CEA, carcinoembryonic antigen; LDH, lactate dehydrogenase
Abdominal ultrasound showed cystic formations throughout the hepatic parenchyma which seem to communicate with the intrahepatic bile ducts. There is no calculus or liver nodule. Magnetic resonance imaging (MRI) (Figure 1) confirmed the diagnosis of CD. The liver was of normal size, with regular contours with the presence of multiple cystic formations in hyposignal T1 and hypersignal T2, with thin walls. These cystic lesions are connected to and communicate with the intrahepatic bile ducts.
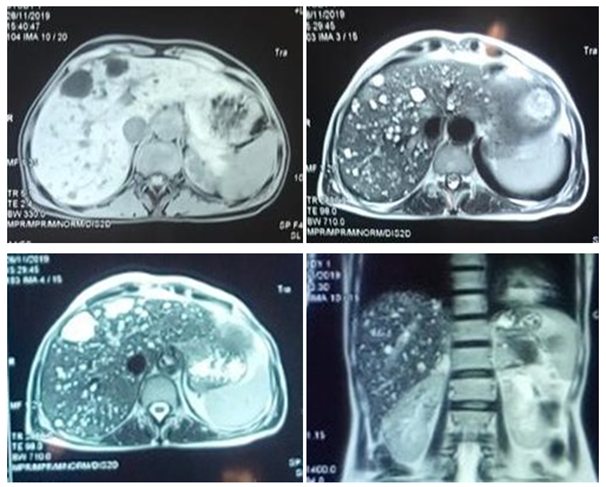
Figure 1 Magnetic resonance imaging showing ectasias of the intrahepatic bile ducts and communication with the bile branches.
Liver biopsy was not performed because the procedure would bring more risks than benefits. Currently, the patient continues her regular clinical follow-up in consultation. She has no major clinical manifestations that affect her daily life. The prognosis for her illness is favorable at the moment, since the patient has no complications including no cholangitis and no signs of portal hypertension or malignancy.
Caroli's disease is a rare autosomal recessive inherited congenital disease. Rare cases of autosomal dominant transmission have been reported.4 Its incidence is estimated at 1 per million of the population.5 It is associated with incomplete and defective remodeling of the embryonic ductal plate.6,7 It is usually diagnosed in childhood or adolescence,4 but can be diagnosed in adulthood.8–10 No sex predominance has been reported.11
The clinical symptomatology of CD is not specific. Patients may be asymptomatic or have symptoms such as jaundice, right upper quadrant pain or fever.12 Intrahepatic ductal ectasia predisposes to the stagnation of bile, which can lead to the formation of stones and predisposes to repeated cholangitis which can be complicated by serious infections such as liver abscess or septicemia.13,14 Chronic inflammation due to gallstones in the intrahepatic ducts can lead to secondary biliary cirrhosis with its complications such as portal hypertension.14 Liver function tests in CD may be normal or there may be reversible increases during episodes of cholangitis.15 The level of alkaline phosphatase and direct bilirubin may be increased. The level of transaminases can be normal as it can be high and reflect a progressive hepatic fibrosis in case of Caroli syndrome.14
The final diagnosis is confirmed by histopathology, but current non-invasive imaging tools, including ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) are considered to be the first-line diagnostic modalities.16 The ultrasound shows saccular or spindle-shaped cystic dilations predominant around the hepatic hilum without any underlying obstacle and which communicate with the rest of the biliary tree. It is a very good technique for visualizing intrahepatic lithiasis. Doppler ultrasound or multi-slice CT also enable visualization of the pathognomonic “dot sign”, which represents a portal or arterial branch at the periphery of or within a pseudoseptation running through a cyst.17 Magnetic resonance imaging (MRI) is a non-invasive technique that currently presents the most specific tool for diagnosing CD. It demonstrates cystic structures of variable size which communicate freely with the biliary tree as well as the "dot sign" and intrahepatic stones.18 Caroli syndrome differs from CD by the presence of smaller cystic structures (<2cm) connected to the bile ducts with periportal fibrosis.19
The management of CD depends on the symptomatology of the patient and the extent of biliary abnormalities.10 Cholangitis can be managed with antibiotics. In the case of cholestasis with intrahepatic lithiasis, the prescription of ursodeoxycholic acid is an option.17,20 Surgical resection by lobectomy is recommended in case of localized disease in a lobe.21 It reduces the risk of degeneration into cholangiocarcinoma which to be as high as 100-fold greater in patients with CD than in the general population.22 However, in cases of diffuse disease and in case of suspected malignant transformation, liver transplantation remains the treatment of choice.23 In case of biliary obstruction, an endoscopic sphincterotomy, radiological or surgical drainage may be applied.15
Caroli disease poses a double diagnostic and therapeutic problem. The rarity of the disease, the absence of specific clinical signs and the frequent association with gallstones make diagnosis difficult. Treatment of diffuse forms is problematic and requires liver transplantation given the risk of septic complications, secondary biliary cirrhosis and degeneration.
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript
None.
None.

©2020 Lemfadli, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.