MOJ
eISSN: 2381-179X


Case Report Volume 14 Issue 1
Imaging Center Diagnostic of Breast, Zambrano-Hellion Hospital, Tecnológico de Monterrey, Mexico
Correspondence: Yazmin Olivares-Antúnez, Imaging Center Diagnostic of Breast, Zambrano-Hellion Hospital, Tecnológico de Monterrey, Nuevo León, Mexico
Received: January 21, 2024 | Published: January 29, 2024
Citation: Olivares-Antúnez Y, Dávila-Zablah YJ, Garza-Montemayor ML. Breast pseudoaneurysm: two case reports. MOJ Clin Med Case Rep. 2024;14(1):5-10. DOI: 10.15406/mojcr.2024.14.00450
Breast pseudoaneurysm (PSA) is known as pulsatile or communicated hematoma or false aneurysm. The incidence is 0.05%-0.5%. PSA can be spontaneous or secondary to interventional breast procedures, surgery, penetrating chest trauma, local infection, and burns. Meanwhile, spontaneous PSAs occur in elderly patients, with atherosclerosis or with coagulation therapies. We present two cases of PSA, the first case is a 29-years-old female and a 49-years-old female, who came for a check-up due to a recent a pulsatile mass after a breast core needle biopsy. Faced at hypoechoic mass is important to use color Doppler to rule out the diagnosis of PSA. Because of the low incidence, guidelines for treatment or a standard conduct are not established. However, it is important to set up management in order to avoid further complications.
Keywords: Pseudoaneurysm, ultrasound, mammography, magnetic resonance, core needle biopsy
Screening methods have allowed the early detection of benign and malignant breast tumors. For histological confirmation, minimally invasive techniques are used, such as percutaneous biopsies with a core needle or vacuum assisted closure (VAC) system by stereotactic or ultrasound- guided. Complications of these procedures are hematomas, bleeding and pseudoaneurysms. Pseudoaneurysm (PSA) is known as pulsatile or communicated hematoma or false aneurysm. PSA is a rare entity and guidelines for treatment are not established. The diagnosis is made by imaging and the purpose of this work is to describe the characteristics of PSA by mammography, ultrasound, and magnetic resonance (MR) to make an early detection. Also, we conducted a review in the literature regarding all the possible treatments in order to avoid further complications of PSA.
29-years-old woman has been diagnosed with carcinoma by core needle biopsy recently performed in the left breast. She attends our department due to a pulsatile and palpable mass in the site of the biopsy. By mammography, in craniocaudal (CC) and mediolateral Oblique (OML) projections, the breast tissue is the heterogeneously dense (category “C” of the American College of Radiology, 2013). In the left breast, exists a focal asymmetry associated with residual cluster of amorphous microcalcifications and architectural distortion, localized in the middle third of the upper outer quadrant that coincides with the site of the biopsy. Adjacent to these findings, at the superior region, a new nodule is observed. This nodule is isodense, round and with microlobulated margins, which corresponds to the palpable area which is signaling by triangular tissue marker. In the ipsilateral axillary region, an anormal lymph node is seen (Figure 1). In the MLO and electronic magnification of MLO projection, the nodule and the residual calcifications are clearly visible (Figure 2). By Digital breast tomosynthesis (DBT) in CC and MLO projections, the margins of the nodule are well defined, and the architectural distortion is confirmed (Figure 3 and 4). By ultrasound, in the left breast, we find a 1.3 cm, circumscribed, oval, hypoechoic nodule with thin wall and echogenic ring, located at 2 o'clock position, 5 cm from the nipple, nearby the site of the biopsy. Color Doppler demonstrates bidirectional vascularity within the nodule termed “the yin-yang sign” (Figure 5). Subtraction MR imaging shows spiculated, irregular, and heterogenous enhancement nodule, which corresponds to the malignant tumor. Adjacent to the primary tumor, the T2-weighted sequence shows a round and circumscribed nodule. This nodule and its nutrient vessel are isointense to water (Figure 6). In this case, left mastectomy and sentinel lymph node biopsy is indicated. The primary tumor and PSA are surgically removed. At the present time, the patient has metastatic lesion in the brain.

Figure 1 Craneocaudal and Mediolateral Oblique projections. The breast tissue is the heterogeneously dense (category “C” of the American College of Radiology, 2013). In the left breast, exists a focal asymmetry associated with residual cluster of amorphous microcalcifications and architectural distortion, localized in the middle third of the upper outer quadrant, that coincides with the site of the biopsy (black arrow). Adjacent to these findings, at the superior region, a new nodule is observed (white arrow). This nodule is isodense, round and with microlobulated margins, which corresponds to the palpable area which is signaling by radiolucent triangular tissue marker. In the ipsilateral axillary region, an anormal lymph node is seen (head arrow).
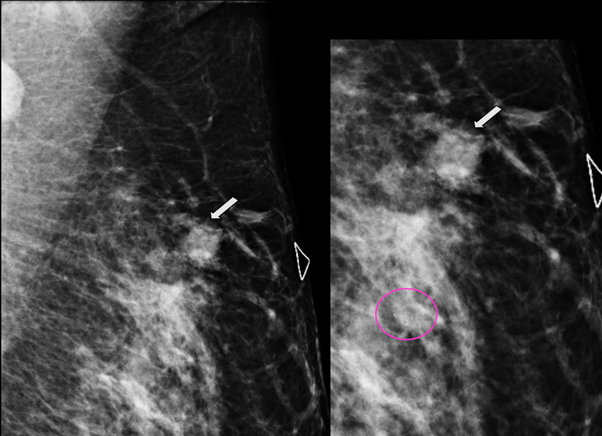
Figure 2 MLO and electronic magnification of MLO projection. The nodule (arrow) and the residual calcifications (circle) are clearly visible.

Figure 3 Digital breast tomosynthesis (DBT) in MLO projection. The margins of the nodule are well defined (white arrow) and the architectural distortion is confirmed (black arrow).

Figure 4 Digital breast tomosynthesis (DBT) in CC projection. The margins of the nodule are well defined (white arrow) and the architectural distortion is confirmed (black arrow).

Figure 5A) Gray-scale ultrasound image. In the left breast, a 1.3 cm, circumscribed, oval, hypoechoic nodule with thin wall and echogenic ring is seen at 2 o'clock position, 5 cm from the nipple, nearby the site of the biopsy. B) Color Doppler image. The nodule demonstrates bidirectional vascularity within it, termed “the yin-yang sign”.
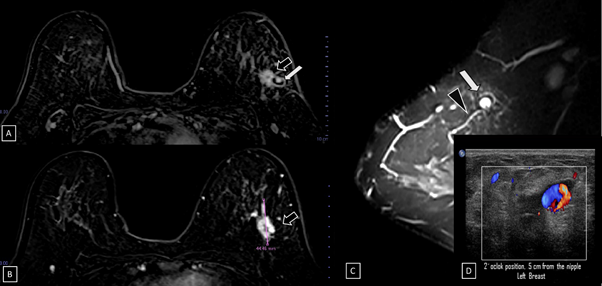
Figure 6 Subtraction MR imaging.
A) and B) A spiculated, irregular, and heterogenous enhancement nodule is observed, which corresponds to the malignant tumor (black arrow).
C) T2-weighted sequence image. Adjacent to the primary tumor, a round and circumscribed nodule is identified. This nodule (white arrow) and its nutrient vessel (black head arrow) are isointense to water.
D) Color Doppler image. The yin-yang sign is shown withing the nodule.
49-year-old women was diagnosed with palpable and suspicious nodule of right breast. Therefore, a core needle biopsy by ultrasound-guided was performed. The pathological report reveals a benign nodule. Two days after percutaneous biopsy, she attends to our breast center due to a new and palpable nodule in the right breast. In the craniocaudal and electronical magnification projections a round, isodense and circumscribed nodule is found in the anterior third of outer quadrant of the right breast (Figure 7). By ultrasound, a round, circumscribed, thin-walled nodule, with internal echoes is visualized in the right breast at 11 o'clock position, 8cm from the nipple, adjacent to the biopsy site. Color Doppler shows the Yin-Yang sign at the center of the nodule (Figure 8). We explained to the patient about the therapeutic options and collateral effects; however, she rejected treatment for PSA. In subsequent mammography projections, the PSA starts to appear like a hypodense nodule with increased microcalcifications within it, until a microcalfication completely covers the nodule. Meanwhile, on gray-scale ultrasound images, periodically, the nodule is filled with microcalcifications. Using color Doppler, the nodule is seen with less internal vascularity until it becomes avascular. This description corresponds to a PSA with spontaneous thrombosis after 7 years of the diagnosis (Figure 9).

Figure 7 A) Craniocaudal and B) electronic magnification projections. A round, isodense and circumscribed nodule (arrow) is found in the anterior third of the outer quadrant of the right breast.

Figure 8 A) Gray-scale ultrasound image. A round, circumscribed, thin-walled nodule, with internal echoes is visualized in the right breast at 11 o'clock position, 8cm from the nipple, adjacent to the biopsy site. B) Color Doppler image. The Yin-Yang sign is present at the center of the nodule.

Figure 9 Craneocaudal projection, gray-scale ultrasound and color Doppler images.
A) In the year 2014, the PSA is a dense, round nodule with circumscribed margins (arrow). In the Gary-scale ultrasound a tiny microcalcification is seen within the nodule. PSA is round, hypoechogenic and with circumscribed margins. Using color Doppler, aliasing phenomenon is observed because the vascularity shows high velocity flow, which reflects the arterial nutrition.
B) In the year 2018, the PSA became less dense respect to the previous images (arrow). In the Gary-scale ultrasound, the nodule presents peripheral microcalcifications. Using color Doppler, the nodule is avascular and internal vascularity is demonstrated at the nutrient artery of PSA.
C) In the year 2020, coarse microcalcification is seen within the hypodense nodule (arrow). In the Gary-scale ultrasound, the nodule shows a echogenic rim. Using color Doppler, low vascularity is seen in the PSA.
D) In the year 2022, a greater coarse microcalcifcations is seen within the hypodense nodule (arrow). In the Gary-scale ultrasound, the nodule shows a coarse microcalcifications that creates posterior acoustic shadowing. Using color Doppler, the PSA is avascular which is thrombosed.
Pseudoaneurysm (PSA) is known as pulsatile or communicated hematoma or false aneurysm. Its incidence is 0.05%-0.5%.1-20 PSA is produced by a local trauma generating transmural disruption of the vascular wall and the arterial pressure of the blood cuts up the adjacent tissues, creating a sac that communicates with the lumen of the vessel. This sac is surrounded by 2 layers, the media, and the adventitia. The PSA also can be delimited by perivascular tissue, clots or a layer of reactive fibrosis that restricts the arterial rupture. The channel between the extravascular space and the vessel lumen remains permeable due to the pulsatile and expansive nature of the PSA. The spontaneous PSA occurs in elderly patients with atherosclerosis or patients with coagulation therapies. PSAs can also be secondary to surgery, penetrating chest trauma, local infection, and burns.2-5,7,8,10,12,13,15-17,19,20
PSA is a rare complication of interventional breast procedures (core needle or vacuum-assisted biopsy). Some researchers have been reported PSA in which the superficial thoracic artery and the internal mammary artery were affected due to iatrogenic trauma.2,4,5,8 The clinical presentation appears immediately or after days, weeks or even months since the procedure. The patients have a painful, palpable, and pulsatile mass which recently appeared at the site of the previous biopsy. Some cases are asymptomatic.5,10,13,18 The diagnosis of PSA is based only on imaging studies. An early detection is important to avoid further complications and establish treatment. The complications of pseudoaneurysm are rupture, massive bleeding and arterial thrombosis.17,19
In mammography, the PSA is isodense, circumscribed, round or oval nodule or mass, near a vascular tract, which may resemble an intramammary lymph node.10,12,20 In ultrasound images, the PSA is seen as a new, saccular, hypoechoic, or anechoic, oval, circumscribed, thin-walled nodule or mass, with an echogenic ring that is pulsatile and is located near or in the site of biopsy. PSA can measure 1cm to 3cm and connects with the artery that originates it.3,4,6,9,12,15,16,17,18,20 After 3 weeks, PSA is heterogeneous mass with an anechoic center and hyperechogenic ring. Three months later, PSA appears with echogenic material inside the nodule and with absent of vascularity using color Doppler due to spontaneous thrombosis.1,4,7,9,10,12,17,18,20 Color Doppler has a diagnostic certainty of 95% for PSA.9,10,16
PSA, attributable to its pulsatile nature, maintains a bidirectional arterial flow through the vascular sac; During the high intra-arterial pressure of systole, the blood flow is anterograde towards the pseudoaneurysm and during the diastole the flow direction is retrograde, causing a turbulent or swirling flow in the non-thrombosed portion of PSA, forming the typical Yin-Yang sign.1-4,6,9,10,12,14-20 In angiotomography, PSA appears as a hypodense saccular mass, with a soft tissue wall, that is communicated to the artery that origins it. The PSA is associated with central enhancement. Some PSAs do not enhance and appear hypodense and homogenous.10
In MR, PSA is a round, circumscribed mass. On T1 and T2-weighted sequences, it appears hyperintense because blood containing. After intravenous administration of gadolinium, PSA and nutrient artery present homogeneous enhancement.10 On imaging studies, differential diagnoses of breast PSA include complex cyst, true aneurysm, hematoma and arteriovenous fistula. The complex cyst is a heterogeneous nodule with a solid component that show absent or minimal internal flow with color Doppler and there is not any communication with an arterial vessel. The true aneurysm is a dilation that affects the 3 arterial layers (intima, media and adventitia) and is less common than the PSA.3,4,8,10,12,13,17,19
The hematoma is seen on mammography like a focal asymmetry. The hematoma does not appear as an oval or irregular mass and tends to calcify. In the acute phase, the ultrasound reveals a hyperechoic collection that becomes a complex nodule with solid and cyst areas. Using Doppler color there is absence of vascularity of the mass. Subsequently, the hematoma completely disappears or degenerates into fat necrosis. On MRI, at the early phase it is hyperintense on T1-sequence, hypointense on T2-sequence and does not show enhancement.10 Arteriovenous fistula (AV) has a large nutrient artery and a large vein. A-V fistula is presented as pulsatile hematoma with audible murmurs. Using color and spectral Doppler, the venous and arterial flow pattern confirm the diagnosis.21
In our Institution, the incidence of PSA is 0.03% (7160 performed procedures), having only two cases since 2006 which is similar to the incidence reported in the literature. The 2 cases that we presented correspond to pseudoaneurysms as a complication of a core needle biopsy; the diagnosis was made through clinical symptoms and color Doppler findings. On imaging, it presented with the most frequent characteristics described in the literature, such as an oval, hypoechoic mass with a Yin-yang sign. In the first case, the diagnosis of carcinoma was made, and the treatment was surgery resection of the neoplasia and the PSA. In the second case, the therapeutic options and side effects were explained to the patient, however, she refused treatment for PSA and after 7 years, we show by imaging studies how the PSA is spontaneously thrombosed.
There are no guidelines for the treatment or a gold standard treatment for breast PSA. It is necessary to take into account the size, location, risk of complications and comorbidities of the patient for treatment.4,13,15 One option is conservative treatment that consists of ultrasound-guided compression for 20 to 60 minutes on the neck of PSA. Follow-up by ultrasound is performed for 2-7 days to ensure and document spontaneous thrombosis. The spontaneous thrombosis is associated to the size of the PSA, the diameter of the neck, and the anticoagulant status of the patient.9,11,19,20
The size of PSA neck is fundamental to know if the patient will benefit from percutaneous treatment. If the neck is narrow, management with compression or prothrombin percutaneous injection may be considered. A relative contraindication for the administration of prothrombin or anhydrous alcohol is a diameter of the arterial channel of PSA greater than 0.8cm. Cai et al. found a PSA that measures 6.7X3.5X5.3cm, with an arterial diameter of 0.7cm. In this case, they decided to use microwave ablation to seal the blood vessels of PSA, afterwards they eliminated the hematoma with the vacuum system guided by ultrasound.2,6
Gan et al. found a PSA that measures 25x30 mm and originates from the internal mammary artery. US-guided percutaneous thrombin injection (UGTI) was performed to the patient. Approximately 15 units of thrombin were injected into the cava of the pseudoaneurysm resulting in immediate thrombosis of the sac. The ultrasound of the breasts showed no blood flow in the cavity after the procedure.5
Vavala et al. reported a case of PSA of the intercostal branch of the internal mammary artery. The PSA measures 8x7x4mm. It was managed with ultrasound-guided compression for 20 minutes and 1 month later the PSA was still permeable, and they performed embolization with coils, resulting in complete thrombosis of the PSA without any complications.1 Available treatments include surgical resection, surgery for ligation, minimally invasive percutaneous embolization or endovascular coiling. Open surgery is the last treatment option when the minimally invasive treatment modalities fail.1,5 Using PubMed, we found 48 cases of secondary breast PSA published since 1995 to nowadays and we made a review of age presentation, clinical manifestation, the diameter measure, established treatment and follow-up of each reported cases of literature. Table 1 summarizes the date.
|
Author |
Age of the presentation |
Clinical manifestation |
Major diameter (cm) |
Treatment |
Follow-up |
|
Russell et al.22 |
45 years-old |
Bleeding and hematoma |
0.9 |
Follow-up and monitoring |
Without complications |
|
Ribeiro Filho et al.23 |
51 years-old |
Bleeding, hematoma and palpable and pulsatile mass |
2.9 |
Surgical resection |
Without complications |
|
Pesce et al.9 |
36 years-old |
Bleeding |
1.3 |
Follow-up and monitoring |
Without complications |
|
Azorín Samper et al.15 |
47 years-old |
No clinical manifestations was mentioned. |
0.6 |
Surgical resection |
Without complications |
|
Vignoli et al.14 |
62 years-old |
Palpable and pulsatile mass with pain. |
2 |
Surgical resection |
Without complications |
|
Lee et al.17 |
64 years-old |
Palpable and pulsatile mass |
5 |
Surgical resection |
Without complications |
|
Sasada et al.24 |
51 years-old |
Bleeding and pulsatile and palpable mass |
1.9 |
Surgical resection |
Without complications |
|
Farrokh et al.25 |
42 years-old |
Palpable and pulsatile mass with pain. |
2-3 |
Ultrasound‐guided compression |
Complete trombosis. |
|
Dixon et al.26 |
46 years-old |
Bleeding hematoma and pulsatile and palpable mass. |
3 |
Surgical resection |
Without complications. |
|
Erdil et al.27 |
41 years-old |
Bleeding hematoma and pulsatile and palpable mass. |
0.2 |
Surgical resection |
Without complications. |
|
Buck et al.28 |
74 years-old |
Palpable and pulsatile mass |
3 |
Injection of protrombin by ultrasound-guided |
Complete trombosis. |
|
Bitencourt et al.29 |
46 years-old |
Bleeding and hematoma |
------- |
Ultrasound‐guided compression |
Complete trombosis. |
|
El Koury et al.30 |
50 years-old |
Bleeding and palpable and pulsatile mass. |
2 |
Without treatment, the patient rejects surgical resection. |
Spontaneous regression. |
|
Bazzocchi et al.31 |
42 years-old |
Bleeding and palpable and pulsatile mass. |
1.6 |
Percutaneous injection of Hydrous etanol. The patient rejects surgical resection |
Complete trombosis. |
|
Chormy et al.32 |
44 years-old |
Bleeding and hematoma. |
0.8 |
Ultrasound‐guided compression |
The compression failed and the option was for surgical resection. |
|
McNamara et al.33 |
70 years-old |
Bleeding |
1 |
Ultrasound‐guided compression subsequently protrombin injection. |
Complete trombosis. |
|
Swain et al. 3 |
65 years-old |
Bleeding |
1.7 |
Surgical resection |
Without complications. |
|
Zhenyu et al.34 |
48 years-old |
New mass with pain. |
6.7 |
Microwave ablation |
Without complications. |
|
Omisore et al.4 |
51 years-old |
Painful mass |
2 |
Conservative treatment |
Spontaneous thrombosis after 3 months. |
|
Igram et al.20 |
24 years-old |
Palpable mass |
2.5 |
Percutaneous injection of 15 unit of protrombin by ultrasound-guided. |
Complete trombosis. |
|
Vavala et al.1 |
30 years-old |
New palpable lump |
0.8 |
Compression and embolization with coils |
Complete trombosis. |
|
De Souza et al.10 |
29 years-old |
New mass |
0.8 |
Surgical resection of myxoid fibroadenoma and PSA. |
Without complications. |
|
Binh et al. 11 |
35 years-old |
Rapid grow-up mass |
4.5 |
Compression, injection of tranexamic acid and embolization with Cyanoacrylate-Lipiodol injection therapy. Fr sonde was inserted to drain the hematoma. |
Without complications. |
|
Gity et al.35 |
29 years-old |
Palpable and painless mass |
1.2 |
Conservative treatment. |
Spontaneous thrombosis after 2 months. |
|
Neamonitou et al.13 |
51 years-old |
Incidental finding by ultrasound. |
2 |
Surgical resection of the PSA and the primary nodule |
Without complications. |
Table 1 Treatment of PSA according to the reported cases in literature
Breast pseudoaneurysm is a rare complication of interventional procedures. Prior biopsy, we recommend planning the access and the path of the core needle. It is important to identify the vessels that are near the target microcalcifications by mammography or to localize the vessels around the target mass by using color Doppler to reduce the risk of PSA. In case of bleeding, only compression should be applied until hemostasis. If spontaneous thrombosis does not occur there are treatment options available (Table 1). We must suspect a PSA when there is a pulsatile mass in a patient with recently performed biopsy and hematoma formation during the procedure. Gray-scale ultrasound and color Doppler is needed to confirm or rule out the diagnosis of PSA. The color Doppler will show the typical findings in case of PSA, without further imaging studies. We recommend multidisciplinary consensus for PSA which does not show immediately spontaneous thrombosis considering the measure of PSA neck for an adequate management.
None.
All the authors report no conflicts of interest for this article.

©2024 Olivares-Antúnez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.