MOJ
eISSN: 2381-179X


Case Report Volume 6 Issue 1
1Department of Internal Medicine/Cardiology, Michigan State University, USA
2Department of Internal Medicine &Pediatrics, Western Michigan University Homer Stryker School of Medicine, USA
3Department of Cardiology, Borgess Medical Center, USA
Correspondence: Jagadeesh K Kalavakunta, Department of Internal Medicine/Cardiology, Borgess Medical Center, Kalamazoo, Michigan, USA
Received: January 08, 2017 | Published: February 1, 2017
Citation: Lule E, Achike O, Agrawal Y, et al. Antiarrhythmic medication induced multi-organ dysfunction. MOJ Clin Med Case Rep. 2017;6(1):27-28. DOI: 10.15406/mojcr.2017.06.00150
Propafenone is a Class 1 C drug indicated for reduction of episodes of paroxysmal atrial fibrillation. There is a paucity of literature to guide the escalation of propafenone in a patient who has been on long-term therapy. Multi-organ toxicity following dose escalation within the recommended therapeutic dose range appears to be a rare event. We report a patient who presented with severe systolic heart failure six weeks after propafenone dose escalation. We discontinued propafenone therapy and the patient had full clinical and echocardiographic recovery.
Keywords: propafenone, cardiotoxicity, hepatotoxicity, dose escalation
AF, atrial fibrillation; ECG, electrocardiogram; TTE, transthoracic echocardiogram; INR, international normalized ratio; SVT, supraventricular tachycardia
Propafenone used in the treatment of atrial fibrillation and supraventricular tachycardia is a class IC anti-arrhythmic medication which is generally safe at when administered in the thera peutic range. Under rare circumstances, toxicity involving the cardiovascular system can occur.
A 77-year-old Caucasian female with history of chronic atrial fibrillation (AF) on propafenone 150mg three times daily was admitted for uncontrolled ventricular rates and dyspnea on exertion. Physical exam was relevant for irregularly irregular heart rate at 145 beats per minute (bpm). Her initial electrocardiogram (ECG) revealed atrial fibrillation at a rate of 145 beats per minute (Figure 1). Labs and transthoracic echocardiogram (TTE) were in normal limits. Her propafenone was increased to 225mg three times daily which did not improve her AF. She was then successfully electrically cardioverted to normal sinus rhythm two days later and was discharged on this increased dose of propafenone.
She was admitted 6weeks later with dyspnea and palpitations. Physical exam revealed irregularly irregular heart rate. Labs showed elevated liver enzymes (AST- 1049U/L, ALT-577U/L) and international normalized ratio (INR) of 23. Labs for viral hepatitis and autoimmune hepatitis were negative. ECG revealed AF with heart rate of 111bpm with left bundle branch block (Figure 2). TTE was significant for ejection fraction of 20% and paradoxical septal wall motion. Propafenone toxicity was high on the differential was causing the present clinical picture and it was discontinued along with warfarin which she was on for anticoagulation.
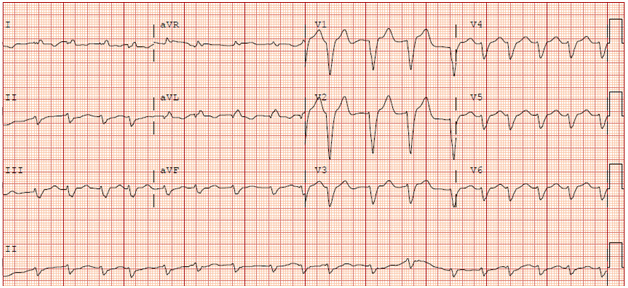
Figure 2 EKG upon readmission 6weeks later. It revealed atrial fibrillation with a heart rate of 111bpm with left bundle branch block.
After discontinuation of the propafenone, she improved clinically with normalization of transaminitis, INR, ECG showing rate of 88bpm with loss of left bundle branch block and TTE showing 60-65% ejection fraction on day 10 of her admission. She was discharged and has been doing well at her outpatient follow up appointments.
Propafenone (2′-[3-(propyl amino)-2-(hydroxy)-propoxy]-3-phenylpropiophenone hydrochloride) is a Vaughn Williams Class 1C antiarrhythmic drug with proven efficacy in reduction of episodes of supraventricular tachycardia (SVT) and AF.1,2 Guidelines on AF management state that propafenone may be initiated as an out-patient if care is taken to ensure the patient has no structural heart disease, no sinus node or atrio-ventricular node disease and no Brugada pattern on EKG.3
Propafenone increased the median free interval without recurrence of arrhythmia to 44days in the ERAFT (European Rythmol/Rytmonorm Atrial Fibrillation Trial) trial,1 a mean of 98days in the PSVT study2 and over 300days in the RAFT (Rythmol Atrial Fibrillation Trial) trial.4 Propafenone has also been shown to be effective in converting patients with recent onset of atrial fibrillation with reported success rates of conversion between 72 and 94%.5,6 A recent meta- analysis found that unlike flecainide, propafenone does not have an adverse effect on mortality and it is not pro-arrhythmic.7
In the study of patients with PSVT or atrial fibrillation, congestive heart failure occurred in 1.9% of patients.2 Reduction in ejection fraction is confined to those with preexisting reduced ejection fraction.8 Liver toxicity is a rare side effect of propafenone. In a review of nine cases reported in literature propafenone toxicity was noted a median of 4weeks after propafenone initiation.9 A nonlinear relationship between dose and steady state concentrations has been noted. This means a large increase in plasma concentration can result from a small dose increase.10 We did not come across recommendations when adjusting the dose of propafenone on patients who have been on chronic therapy. We did however find two other cases in literature of marked reduction in systolic function in patients who had been on long term therapy with propafenone and had undergone a medication dose increase.11,12
Our patient is noteworthy for a few reasons. Her ejection fraction fell from normal to an estimated 20%. From our review of literature, it appears such a precipitous decline in ejection fraction with propafenone dose increases in the usual therapeutic range is rare. The most remarkable finding in her ECG at the time of maximal toxicity (when her QRS interval was 176) is the marked left bundle branch block. This caused marked dyssynchrony noted on her TTE.
We believe that our patient’s cardiac and hepatic dysfunction was caused by propafenone considering the sub-acute worsening of her clinical picture after propafenone dose increase, and subsequent improvement after discontinuation of the medication.
Propafenone is an effective drug in converting patients with recent onset AF and reducing recurrences of AF. Although rare, propafenone toxicity should be considered in patients having symptoms of systolic heart failure and hepatotoxicity soon after a dose increase. Monitoring for three days post dose increase may be insufficient in judging the risk of toxicity. It may be reasonable to review ECGs prior to any exposure to propafenone when making dose adjustments much later.
None.
The author declares no conflict of interest.

©2017 Lule, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.