MOJ
eISSN: 2381-179X


Case Report Volume 8 Issue 3
1Professor of Cardiology, Medical College Hospital, India
2Additional Professor of Cardiology, Medical College Hospital, India
3Former Senior Resident in Cardiology, Medical College Hospital, India
Correspondence: Prabha Nini Gupta, Professor of Cardiology, Medical College Hospital, India,
Received: May 27, 2017 | Published: June 19, 2018
Citation: Gupta PN, Sanjai PV, Koshy AG, et al. An unusual cystic mass in the left atrium/what is it? MOJ Clin Med Case Rep. 2018;8(3):133-138. DOI: 10.15406/mojcr.2018.08.00258
Apart from rheumatic heart diseases there are a few more common causes of mitral regurgitation .Here we report a rare cause of mitral regurgitation that presented as a cystic mass in the left atrium .This enlarged in systole and decompressed to a smaller size in diastole. We also discuss the other causes of cystic masses in the left atrium .Our case was a mitral valve gumma(as diagnosed at histopathology examination ) in a patient with a positive Treponema Pallidum He amagglutination test. (TPHA test).
Keywords: left atrial cystic masses, left atrial cystic myxoma, mitral valve aneurysm, blood cyst, mitral valve gumma, mitral valve disease, mitral valve gumma, cystic masses in the left atrium, echocardiography
Mitral regurgitation is a common valvular disease frequently seen all over the world. While in the tropics and underdeveloped countries rheumatic heart disease and acute rheumatic fever are the common causes of mitral regurgitation, in the elderly and in the more developed parts of the world myxomatous mitral valve disease is the commoner cause of mitral regurgitation. Flail mitral valve after a mitral valve prolapse syndrome is also the most common cause of urgent mitral valve repair. The Mida registry is a source for information on this treatment modality.1 Sometimes however the mitral valve may be too damaged to repair and then mitral valve replacement surgery is performed.
Left sided endomyocardial fibrosis, or dilated cardiomyopathy may also present with mitral regurgitation. This is the pattern in Asians in the age group below 60 years at presentation. However not infrequently other rare aetiologies have to be considered. Therefore we report this case.
The patient
A 35yr old woman came to the cardiology outpatient department with complaints of dyspnoea on exertion and exertional palpitations with a functional class II for the past two years, worsening to functional class III for the past 2 weeks. She was not a diabetic, or hypertensive. She had no significant past medical or surgical history. She had no history of headaches or history of exposure to sexually transmitted diseases. But her husband was a bus driver who was not questioned for history of extramarital sexual contact. (In our state bus drivers and prostitutes are considered to be at high risk for HIV infection) . She had tolerated 2 pregnancies without any significant symptoms six years and eight years earlier.
Past history
She had no history of skin rashes or extramarital sexual contact. She had no history of frequent headaches or polyarthritis suggestive of acute rheumatic fever. She also had no history suggestive of collagen vascular disease, or eye disturbances.
On general examination
The patient was a febrile with a pulse rate of 75 per minute, regular in rhythm and high volume in character. Her blood pressure was 124/80mm of Hg, her mean jugular venous pressure was not elevated and showed normal wave forms. Her physical examination was normal and she had no features of marfanoid habitus.
On precordial examination, her apicalim pulse was in the 5 the left intercostal space in the mid clavicular line and was forceful in character. Her first heart sound was soft in intensity; her second heart sound was normally split with normal intensity of both the aortic and pulmonary components. On auscultation at the apex there was a left ventricular third heart sound and a grade 3/6 pan systolic murmur that was conducted to the axilla.
Her echocardiogram showed mild left ventricular and left atrial dilatation with normal left ventricular systolic function. The mitral valve was morphologically abnormal with the anterior mitral leaflet showing an cystic aneurysmal segment with systolic ballooning and some degree of diastolic collapse but incomplete collapse. (Figures 1-3) (Video 1) (Video 2) The aneurysmal segment showed a classical heart shape which was most evident in the end systolic frame. Colour flow imaging showed severe mitral regurgitation with an eccentric jet, the origin of the jet appeared to be through the anterior mitral leaflet. The transeosopheal echocardiography revealed similar findings (Figure 4) (Figure 5)The aortic valve was morphologically normal but for mild cuspal thickening.
Since the mass attached to the mitral leaflets was cystic we considered all the causes for cystic masses in the left atrium as the differential diagnosis.
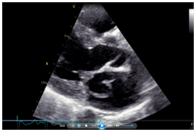
Figure 3 The cystic mass appearing like a heart in the parasternal long axis view on echocardiography.
Haemoglobin-13gm/dl, Total leukocyte count-7500/mm3, Differential count: polymorphonuclear cell count-75%, Lymphocytes-20% Eosinophil count-3%, Monocytes-2%VDRL- positive at 1:32 dilution. TPHA-positive, HIV Elissa-Negative.
Her electrocardiogram showed: Sinus rhythm at a rate of 65/minute .Her mean QRS axis was +20 degrees .Her PR interval was 160 msecs and her QRS duration was 80 msecs. She had no left atrial anomaly or voltage criteria for left ventricular hypertrophy.
On echocardiography. Her left ventricular internal dimensions in systole/diastole were-5.3/3.2cms, her interventricular septal thickness in systole /diastole were 0.9/1.2 cms and her posterior wall thickness in systole /diastole was 0.8/1.2cms and her ejection fraction was 69%. Her anterior mitral leaflet was aneurysmal with ballooning in systole and partially collapsed in diastole and appeared like a cystic mass. Her colour Doppler showed severe mitral regurgitation and mild tricuspid regurgitation. (Her tricuspid regurgitation jet was 20mm Hg. Her aortic valve was trileaf let and normal. She had no aortic regurgitation. Her left atrium was 3.9cms and her aortic diameter was 3.1cms. Her husband also had a positive VDRL and was treated with her.
Differential diagnosis
Since the mass attached to the mitral leaflets was cystic we considered all the causes for cystic masses in the left atrium as the differential diagnosis. On reviewing the literature we considered the following possibilities 1. mitral valve aneurysm, 2.blood cyst of the mitral valve , 3.atypical mitral valve prolapse, 4.cystic atrial myxoma and mitral valve abscess . The other differential diagnoses considered were 5.left atrial hematomas,6.cystic secondary tumours in the left atrium and 7. left atrial cystic thrombi.
Why was it not a blood cyst?-A blood cyst typically remains the same size in both systole and diastole and does not collapse in the various phases of the cardiac cycle. Why was it not a mitral valve aneurysm? A mitral valve aneurysm usually balloons in systole and collapses completely in diastole (due to emptying into the left ventricle in diastole). And is usually associated with severe aortic regurgitation and aortic valve endocarditis. Why was this not a mitral valve abscess? The patient was not toxic and did not show signs of fever and systemic toxicity as should occur in a mitral valve abscess.
It was not a cystic myxoma (we have another case) because the histopathology was not suggestive of a myxoma.
It could have turned out to be an atypical case of mitral valve prolapse where the mitral valve tissue is redundant but the histopathology did not support this diagnosis. Due to the different histopathology, a cystic hematoma and a cystic thrombus was excluded.
Course in the hospital
With a probable diagnosis of mitral valve aneurysm, the patient was referred to the Thoracic surgeon for mitral valve repair. Preoperative evaluation showed a strongly positive venereal disease research lab (VDRL) at a dilution of 1:32, which was confirmed again with a positive TPHA. The patient lacked any other syphilitic stigmata. She was treated with injection Procaine Penicillin 6 lakh units intramuscularly daily for 20 days and taken up for surgery. On the operating table, gross examination of the mitral valve showed the anterior mitral leaflet to be thinned and covered by a flimsy aneurysmal layer which gave way during surgery. (Figure 6) (Figure 7) Hence the mitral valve was replaced and not repaired.
Outcome and follow-up
Histopathological examination of the mitral valve showed infiltration of the mitral valve tissue with numerous macrophages .A granulomatous inflammation with numerous macrophages , epithelioid cells and Langhans+ cells seen to be typical of a gummatous inflammation were seen. (Figures 8) (Figures 9)
The anterior mitral leaflet was excised and mitral valve replacement done with St Jude bileaflet valve as the valve was too damaged to be repaired.
A syphilitic involvement of the mitral valve is very rare but has to be considered in this case. Further since syphilis is making a comeback in the twentieth century we are likely to see more cases like this, so the reporting of this case is important.
Syphilis is a disease that is making a comeback in the 20th century due to the increased incidence of MSM, or men having sex with men.2
Primary and secondary syphilis are easily detected and treated as they occur earlier after contact. Chronologically after these stages, the patient develops latent syphilis which is defined as asymptomatic syphilis, with a positive serology. Early latent syphilis occurs at less than 1 year and late latent syphilis occurs after this. It is important to distinguish between early and late latent syphilis as the treatment varies.
Untreated in the early stages, 30-40% of infected syphilis patients will develop tertiary symptomatic syphilis.3 Commonly tertiary syphilis involves either the cardiovascular system or the nervous system or produces late gummatous disease. Gummas are inflammatory granulomas. This is basically a nodule of inflammatory cells that forms a hyalinised nodule as time passes on. Traditionally gummas can occur almost anywhere. Gummas are benign lesions that can develop in the skin, bone or upper respiratory tract. Gummas of the mouth usually affect the tongue or the soft palate, and at first present as a swelling, and later ulcerate and may even erode into the bone.4,5
Neurosyphilis traditionally affects the meningo vascular tissue so can lead to headaches, neck rigidity without fever or nausea and vomiting and seizures. Cranial nerve involvement or eye involvement in the form of uveitis can occur. An Argyll Robertson pupil may occur.6
Late cardiovascular syphilis usually occurs 5 to 20 years after the initial infection. The common manifestations include-aortitis, aortic regurgitation and aortic aneurysm.4,7,8 The spirochete first localizes in the adventitia and then in the lymphatics. The most common site of aortic involvement is the ascending aorta, this occurs within 50 % of cases. Less common is the involvement of aortic arch, then the descending aorta, and least common of all is the involvement of the abdominal aorta.
The predilection for involvement of the different parts of the aorta depends on the supply of lymphatics. The aortic valve and the coronary ostia are also affected. Involvement of the aortic valve leads to aortic regurgitation. Anecdotal reports suggest that very rarely the myocardium, the conduction system or even the mitral valve can be involved by the gummatous inflammation.9 Mitral valve aneurysms have been described rarely in syphilitic patients, but this has always been in the background of aortic valve disease and aortic regurgitation, where the regurgitant jet striking the anterior mitral leaflet causes structural weakening. In our patient the strongly positive VDRL, confirmed by the TPHA test, the presence of granulomatous inflammation in the mitral leaflet associated with giant cells and the mitral valve aneurysm gives us a strong motive to suggest that this patient indeed had an isolated gumma of the mitral valve.
The treatment of tertiary syphilis of the cardiovascular system
Only surgical treatment is recommended as without treatment, 80% of patients with aortic aneurysm die or those with syphilitic involvement of the aorta die. Antibiotic treatment regimes depend on whether the patient has early latent, late latent syphilis or manifest cardiovascular syphilis.
Echocardiography
Our patient presented as a cystic left atrial mass, and severe mitral regurgitation, so the other causes of left atrial cystic masses should be considered (Videos 1) (Videos 2). A few words about the echocardiographic findings in unusual left atrial masses (cysts).
Though a cystic ‘heart shaped’ mass in relation to the mitral leaflets may be considered pathognomonic for blood cyst of the mitral valve it is unusual for the blood cyst to show any change in morphology between the systolic and diastolic frames.10 In our patient the mass definitely collapsed in diastole. However a partially endothelialised blood cyst can also collapse in diastole and show some degree of ballooning in systole. On careful review of the echocardiogram, the systolic ballooning of the cystic mass and the severe mitral regurgitation jet through the anterior mitral leaflet made us consider the possibility of an anterior mitral leaflet aneurysm. However a mitral valve aneurysm classically shows systolic expansion and complete diastolic collapse. Usually diastolic expansion of the mitral valve aneurysm, in the absence of aortic regurgitation indicates perforation of the aneurysm. So we postulated that our patient had an anterior mitral leaflet aneurysm with a perforation which caused the diastolic expansion and severe mitral regurgitation and with this diagnosis we subjected her to surgery.
Echocardiographic features of mitral valve aneurysms
A mitral valve aneurysm is a distinct echocardiographic entity with an approximate incidence of 0.29% on 4500 TEE examinations.11 Mitral valve aneurysms are most often seen in the background of aortic valve endocarditis and those who develop mitral valve aneurysms usually have pre-existing severe aortic regurgitation. The regurgitant jet striking the anterior mitral leaflet causes structural damage and paves the way for the bacterial seeding by providing a nidus that leads to the weakening of the valve and a mitral valve aneurysm. Mitral valve aneurysms appear on echocardiograms as cystic masses attached to the anterior mitral leaflet. Traditionally this mass collapses completely in diastole.
Uncommonly mitral valve aneurysm may be seen in patients with Marfan’s syndrome, Ehler Danlos syndrome, Mitral valve prolapse syndrome, Hypertrophic cardiomyopathy or acute rheumatic fever or cardiovascular syphilis.
Uematsu et al.,12 have reported a large mitral valve aneurysm, and in their figures show two fenestrations in the mitral leaflets. Their patient presented with acute heart failure during treatment for infective endocarditis and this patient survived after being operated on for both mitral and aortic valve replacement within 3 days of admission.12
When a mitral valve aneurysm is associated with a connective tissue disorder, it may involve either the anterior or the posterior mitral leaflet. Lee and co-worker have described a mitral valvular aneurysm that had not ruptured and that was left to periodic medical follow -up after 6 weeks of antibiotics.13 Their patient had grown cocci in culture. They remarked that a mitral leaflet aneurysm should have a transoesophageal echocardiogram. In their experience they reported that a transoesophageal echocardiogram was 5 times more sensitive in detailing the aneurysms than a transthoracic echocardiogram. As late as 2016, two cases and all the earlier cases have been associated with fever and severe aortic valve endocarditis.13–17 In our patient there was no evidence of aortic valve endocarditis even though the patient had fever. Janardhanan et al.,16 have even shown the images of the patient on three-dimensional echocardiography.16 Of course previous authors have already commented on the incremental value of 3 dimensional echocardiography in cases of mitral valve aneurysms.17 Zarrini18 has published a case of isolated mycotic aneurysm of the mitral valve, not associated with aortic valve endocarditis in 2015. The patient had only streptococcal endocarditis of the mitral valve. Her aortic valve was normal. Our patient had no features of aortic valve endocarditis, nor any positive blood cultures. Obviously as we have reported our patient did not have a mitral valve aneurysm.
Echocardiographic features of blood cysts
Usually blood cysts are cystic masses in the left atrium seen in infants who die within 2 months of age and have had an autopsy.19 These are congenital in origin and have been reported to occur on the mitral valve, in all the chambers of the heart including the right atrium, left atrium or left or right ventricle. The above authors report an asymptomatic adult with a blood cyst .These structures can cause left ventricular outflow tract obstruction, or stroke or coronary obstruction. Blood cysts may be attached to the left atrial wall. These blood cysts can be identified on histopathology. Obviously our patient did not have a blood cyst as the histological picture did not match.
Other causes of cystic masses in the left atrium include hydatid cysts, cystic thrombi, cystic myxomas and even bronchogenic cysts. Some authors Christina Wood et al.,20 report a stromal cell tumour causing a cystic mass in the right atrium. Their patient was a 71 year woman who had an low grade endometrial stromal sarcoma. This tumour produced a cystic mass in the right atrium that was detected by contrast echocardiography and transoesophageal echocardiography. Strangely this tumour was attached to a solid tumour extension in the inferior vena-cava that reached up to the uterine artery. This mass was detected during transoesophageal echocardiography and was missed during routine transthoracic echocardiography. This therefore suggests that whenever an intracardiac mass is found anywhere in the heart, the patient should be subjected to a transoesophageal echocardiogram to study the true extent of the mass.
A cystic thrombus of the left atrium
Ozher et al.,21 report another cystic mass in the left atrium. This was found in a hypertensive women on transeosopheal echocardiogram ,and was attached to the left atrial appendage. As the left atrial appendage is a common site for a thrombus, this was believed to be a thrombus. The patient was treated with intravenous heparin and the whole mass disappeared.21
The another cause of cystic masses in the left atrium are cystic tumours
Cystic tumours can be either primary or secondary. Primary cystic tumours already reported are the cystic rhabdomyomas, cystic liposarcomas and cystic myxomas. Liao report a large cystic myxoma in the left atrium that was initially detected on CT scan and later on echocardiography.22 Another benign cystic cardiac tumour is a lymphangioma.23
Left atrial hematomas
Spontaneous intramural hematomas in the left atrium are very rare, approximately less than 5 cases have been reported till date. They also appear like cystic masses and in the case described, the mass appeared to have a thick capsule- like outer wall .On transoesophageal echocardiogram no flow could be detected inside this mass. It obstructed the outflow from the left atrium but did not obstruct the pulmonary venous inflow. On surgery these masses were found to be intramural hematomas. The wall of the mass had elements of atrial muscle.24
Among the rare causes of cystic masses in the left atrium, isolated mitral valve gumma should be considered and looked for. Apart from appearing cystic and being attached to the mitral valve there are no specific echocardiographic diagnostic features to be looked for. The proof of the pudding is in the serology and the histopathology.
None.
Author declares no conflict of interests. During this study nothing banned by the US FDA has been used.

©2018 Gupta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.