MOJ
eISSN: 2381-179X


Case Report Volume 3 Issue 1
1National Heart Centre, National University Hospital System, Singapore
2Yong Loo Lin School of Medicine, National University of Singapore, Singapore
Correspondence: Chih Chiang Nieh, National University Heart Centre, National University Hospital System, Yong Loo Lin School of Medicine, National University of Singapore
Received: October 07, 2015 | Published: October 24, 2015
Citation: Nieh CC, Sim MA, Poh KK, et al. A rare presentation of churg-strauss syndrome in Singapore associated with elevated cardiac troponin concentration in the absence of acute coronary syndrome or myocardial injury complicated by ischemic bowel and stroke. MOJ Clin Med Case Rep. 2015;3(1):169-171. DOI: 10.15406/mojcr.2015.03.00051
Churg-Strauss syndrome (CSS) is very rare disease. It can be mistaken for other medical conditions upon presentation. Sometimes in the early phase, the only clue is eosinophillia. If treatment is not prompt, vascular complications can occur, resulting in widespread multi-organ involvement. To date, CSS has not been reported to cause a rise in cardiac enzymes in the absence of acute coronary syndrome, myocarditis, pericarditis and cardiomyopathy. We illustrate a case of newly diagnosed CSS in a middle-aged Indian man in Singapore, with no significant history of asthma or chronic rhinosinusitis, who presents with elevated troponin, subsequently complicated by systemic involvement including ocular stroke and ischemic bowel leading to laparotomy and stoma creation.
Keywords: churg strauss syndrome, case report, asian, troponin, cardiac enzymes
CSS, churg strauss syndrome; NSTEMI, non-ST elevated myocardial infarction; MRA, magnetic resonance angiogram; MRI, magnetic resonance imaging; ARA, American rheumatology association
Churg Strauss syndrome is an eosinophilic granulomatosis with polyangiitis it is commonly associated with asthma, eosinophilia and necrotizing vasculitis. Classically it involves three phases, allergic, eosinophillic and vasculitic stage. We present a unique case of Churg Strauss Syndrome associated with raised cardiac troponin levels, opthalmoplegia and gut vasculitis, without the presence of typical respiratory findings of chronic rhinosinusitis and asthma and also illustrating the diagnostic challenges in this condition as it may mimic other medical condition such as stronglyloides infection and acute coronary syndrome.
A 55-year-old Indian man premorbidly, ADL independent, community ambulant with a past history of right ureteric calculi and left ring finger traumatic amputation in July 2013 secondary to accident at work, presented with a three days of intermittent fever of maximum temperature of up to 38.8°C, associated with chills and rigors; occasional non-productive cough, generalized non-specific abdominal colic for ten days, associated with bleeding per rectum upon straining and recent change of bowel habit such as tenesmus; skin lumps over his 4 limbs that appeared 1day ago associated with pruritus, sparing his trunk and face; diaphoresis of three days duration at rest but no chest pain, shortness of breath, palpitation or leg swelling (Figure 1). He also denied weight loss. He is however a heavy smoker of 30 pack-years and has significant family history of ischemic heart disease where his mother underwent a coronary artery bypass graft few years ago and his brother died of acute myocardial infarction at age the age of 50 last year. He has a significant recent travel to Northern Malaysia one week ago. He was not taking any chronic medications. He worked as an oil refinery technician and lived with his wife and children in Singapore; he denied alcohol use. Review of systems was negative for rhinorrhea, diarrhea, vomiting, rash, night sweats and dysuria. It was positive for headache, malaise, myalgias, arthralgias and decreased appetite. He was admitted under Cardiology in view of his diaphoresis and significant family history of ischemic heart disease with the initial impression to rule out acute coronary syndrome.
Physical examination revealed a well-nourished, well-developed man who appeared diaphoretic but was otherwise in no acute distress. He was afebrile at a temperature of 37.3, pulse of 96 beats per minute, respiratory rate of 20 breaths per minute and blood pressure of 138/84mm Hg. Physical examination was otherwise normal other than multiple discrete hyper pigmented firm nodules measuring approximately 1cm by 1cm over his 4 limbs which are fixed to the skin and non-tender. Laboratory examination revealed a white blood cell count of 12.14×109/L (normal, 4.5 to 11×109/L); neutrophils, (61.9%) 7.53×109/L (normal, 2.0 to 7.5×109/L); lymphocytes, (23.5%) 2.85×109/L (normal, 1.5 to 5.0×109/L); monocytes, (7.7%) 0.93×109/L (normal, 0.2 to 0.8×109/L); eosinophils, (6.7%) 0.81×109/L (normal, <0.4×109/L); hematocrit, 34.7% (normal, 36% to 46%); alanine transaminase, 18U/L (normal, 15–38 U/L); aspartate transaminase, 18U/L (normal, 14–38 U/L); alkaline phosphatase, 56U/L (normal, 38–126U/L); C-reactive protein of 103 (normal<10mg/L); Troponin I was elevated at 0.969 (normal<0.039ng/mL). Chest radiograph and Electrocardiography were unremarkable.
In view of the elevated Troponin I, the patient was initially treated as for Non-ST elevated myocardial infarction (NSTEMI). He was immediately started upon dual anti-platelet therapy together with low molecular weight heparin and subsequently underwent an emergent coronary angiogram which showed minor coronary artery disease. Computed Tomography of Pulmonary Arteries was also performed which showed no evidence of pulmonary embolism (Figure 2). The anti-platelet and low molecular weight heparin was then stopped. However, his gastro-intestinal symptoms worsened and he started to spike a temperature once more in the ward. He complained of persistent abdominal pain despite bowel clearance with laxatives. Computed Tomography of Abdomen and Pelvis was performed. The result showed long segment rectal wall thickening seen with peri-rectal fat stranding which was initially thought to be related to aggressive laxative consumption , but malignancy and infection should be ruled out with suggestion of endoscopy; extensive omental fat stranding, most prominent in the upper abdomen abutting the anterior wall of the transverse colon where there was thickening of the anterior transverse colonic wall to rule out possibility of underlying malignancy; peritoneal fat stranding and nodularity seen with minimal ascites; small volume but rounded inferior mensenteric and para-aortic lymph nodes seen.
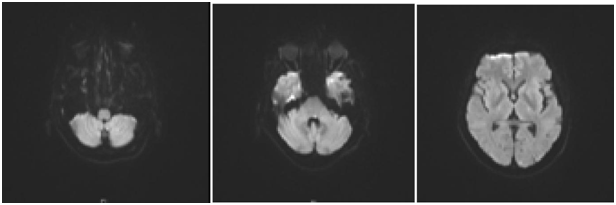
Figure 2 MRA/MRI Brain: Likely acute infarcts in the left cerebellum, occipital lobe, include the left longitudinal fasciculus which would account for the patient’s symptoms.
Celiac lymph adenopathy and non-specific pulmonary nodules were seen. At the same time, his eosinophila also worsened to an absolute level of 6.18. Infectious disease review was sought. The impression at that time was that of possible strongyloides infection and patient was started on Ivertmectin and also covered with IV ceftriaxone. Serology for human immunodeficiency virus was negative. Blood culture was contaminated and grew bacillus species and urine cultures were negative. Stool microscopy was done but cultures were negative. Patient was planned for oesphageal gastroduodenoscopy and colonoscopy, but the procedures were held off in view of patient’s dropping hemoglobin level for which he required 2pints of packed cells for transfusion. Echocardiography and MRI heart were done and results were unremarkable. Subsequently, cultures were performed and revealed to be negative. However, peripheral blood film and skin biopsy performed revealed marked eosinophilia and vasculitis. Serology was negative for stronglyloides. The diagnosis of Churg Strauss syndrome was made and patient was transferred to the care of the rheumatology team and started on cyclophosphamide and prednisolone.
The CT thorax and CT pulmonary angiogram showed marked pulmonary infiltrates and thrombi. There was a thombus in the segmental right lower lobar pulmonary area, with an infracted region in the right lung base. There was also another thrombus in the anterior segmental branch of the left lower lobe pulmonary artery. Subsequently, the patient suffered from opthalmoplegia due to stroke in the ward. The MRI/MRA revealed acute infarcts in the left cerebellum, occipital lobe and left medial longitudinal fasciculus. He also developed gut vasculitis and a perforated transverse colon (Figure 3) (Figure 4). The findings on CT abdomen and pelvis were consistent with that of a small/medium vessel vasculitis. Multifocal ischemic colitis was seen involving the right lateral and posterior walls of the rectum as well as the inferior wall of the mid transverse colon with suggestion of perforation on both sides. The superior rectal artery was thrombosed as well. The patient then underwent emergency laparotomy with resection of small bowel and stoma creation. Post-operatively recovered well and was discharged two weeks after. Currently he is on follow up with rheumatologist, neuroopthalmologist and general surgeon (Figure 5).

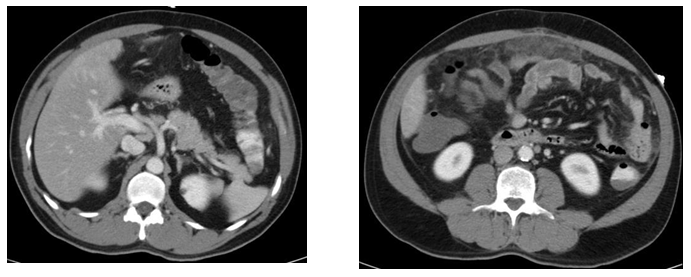
Figure 3 CT abdomen and Pelvis.
Churg strauss syndrome is rarely encountered in the Asian population. The diagnosis of Churg Strauss is not straightforward, with findings varying according to the anatomical site involved and phase of the condition. Both the Lanham’s and more recently, the American Rheumatology Association (ARA) criteria (requires 4 out of 6 findings) are commonly used in the diagnosis of Churg Strauss syndrome. However, these criteria have yet to be validated in an independent patient population. These diagnostic criteria are described in the Table 1.1,2 Churg Strauss Syndrome is characterized by 3 clinical phases the prodromal phase, eosinophilic phase and vasculitic phase. The prodromal phase is typically associated with atopy, asthma and allergic rhinitis. Eosiniphilia and eosinophilic infiltration of the organs (commonly the lung and GI tract) occur during the eosinophilic phase.3 The final phase is the vasculitic phase which is associated with systemic vasculitis of the small and medium vessels; there is usually vascular and extravascular granulomatosis.
Lanham's criteria1 |
American rheumatology association criteria2 |
Asthma |
Asthma |
Peak Peripheral Blood Eosinophil Count >1.5 |
Eosinophilia >10% |
X106cc |
Neuropathy |
Systemic Vasculitis Involving Two or More |
Nonfixed Pulmonary Infiltrates |
Extrapulmonary Organs |
Paranasal Sinus Abnormality |
Extravascular Eosinophils |
Table 1 Diagnostic criteria for churg strauss syndrome
Multiple organ systems can be affected in the vasculitic phase. A rash is common in up to 70% of patients,4 subcutaneous nodules were present in this patient as well. Neuropathy, cerebral vasculitis, pulmonary eosinophilic infiltration, gastrointestinal involvement and cardiac involvement are other possible complications.5 In terms of treatment, Churg Strauss Syndrome is strongly responsive to corticosteroid therapy.6 Thus, prompt diagnosis and treatment should be carried out to avoid the associated complications. Our patient presents atypically due to the lack of prodromal phase. He had no asthma, allergic rhinitis or atopy, apart from the occasional cough. He does not fulfill the Lanham’s criteria, although he meets the ARA criteria for diagnosing Churg Strauss Syndrome. Furthermore, there were raised Troponin I in the absence of cardiac damage including acute coronary syndrome, eosinophilic myofibrosis as shown on MRI, cardiomyopathy, cardiac tamponade, coronary vasculitis, acute pericarditis or pericardial effusion. This is highly unusual and suggests that there could be other underlying causes for raised cardiac enzymes in such patients. Hence, in cases with high index of suspicion, perhaps Churg Strauss could be more strongly considered as a possible diagnosis in patients presenting with similar findings.
The fact that CSS is rare makes the diagnosis rather challenging. Thus prompt evaluation and diagnosis of CSS is necessary to commence definitive treatment so as to avoid life-threatening vascular complications such as ischemic bowel, cerebral vascular accident.
We would like to thank everyone, including the rheumatology team, the cardiology team and the surgical team, who have assisted in the management of this patient.
The author declares no conflict of interest.

©2015 Nieh. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.