MOJ
eISSN: 2572-8520


Review Article Volume 2 Issue 3
1INAT, Tunisia
2INRGREF, Tunisia
3LERMAB, France
Correspondence: Mohamed Tahar Elaieb, INRGREF: Rue H?diKarray, BP: N
Received: February 23, 2017 | Published: March 20, 2017
Citation: Khouaja A, Elaieb MT, Edgar SA, et al. Comparative study of some carbonization process parameters of nine eucalypt woods from hajeblayoun arboretum in Tunisia. MOJ Civil Eng. 2017;2(3):103-106. DOI: 10.15406/mojce.2017.02.00035
The objective of this work is to study the calorific power of nine eucalypt woods from central Tunisia for carbonization process. The ignition temperature, the calorific power of each species and the gas exhaustion during pyrolysis were measured and their average values were compared. The calorific values of the wood of these species varied between 4017 and 4541Kcal/kg. The ignition temperature ranged between 252 and 390°C. The combustion temperature of the samples within the calorimeter was between 463°C and 575°C. The percentage of wood ash after combustion was between 1.0 to 2.2%. The analysis of the experiments conducted during 2 consecutive years showed that certain species were similar while others were significantly different.
Keywords: forest species, temperature, eucalyptus, high calorific capacity
According to the International Energy Association the renewable energy from biomass is the most used in the world. It is the cheapest and always available. The biomass is either used directly as wood energy source, compressed as pellets or carbonised to be used as charcoal. It represents a great economic importance on a worldwide scale. Indeed, wood energy (wood and charcoal included) roughly covers 10% of the energy needs. It provides thus twice more energy than the nuclear power.1
As a source of energy, wood accounts for 5.4% of the total energy used in the world, but with important variations between areas (on average 0.7% in the industrialized countries and 20% in the developing countries.2 However, the overexploitation of the forests generated an environmental imbalance caused by the cutting of forest trees for industrial and energy uses. Thus, certain human activities and various incomplete combustions, carbonization, forest fires, etc cause discharges of nitrogen oxide, carbon monoxide and other noxious gases to the atmosphere and negatively contribute to climatic warning.3 In order to such effects preserve the environment, Tunisia sought the reforestation strategies by mean of using eucalyptus species for energy uses and for its fast growing properties for maximum biomass production in order to meet the local needs. Within this framework, a national strategy of afforestation started since 1988, with the important reforestation objective with an aim of reaching at the year a 2020 rate of national afforestation of 15%.4
The range of the species used covers exotic gasolines such as, theacaciaand autochtones gasolines like the maritime pine, the stone pine, the Aleppo pine, the cypress and various leafy and resinous gasolines but also the Eucalyptus.5,6 Eucalyptus wood species are native to Australia and they were introduced in Tunisia since 50’sby their implantations in bioclimatic arboretums across the country, from centre with a wet climate to the Saharan areas.7,8 These fast-growing species were adapted themselves very well to the Tunisian climate. Now, they tend to become invasive wood species which need to be economically valued. The objective of this work is to evaluate the energy performances of nine species of Eucalypti from the arboretum in the central Tunisia under an arid climate. Moreover, one will compare the results obtained during two consecutive years and data can be used to develop wood thermo degradation mathematical model and apply for industries.9-12
Material
The experimental material concerned nine eucalyptus wood species from the arboretum of Hajeb Layoun :these species were Eu. Oleosa; Eu. Gilli; Eu. Brevifolia ; Eu. Stricklandii ; Eu.Largiforens ; Eu. Patellaris; Eu. Dumosa;Eu. Salmonophloia et Eu. Brockwayi. Disks were cut from the stem at breast height for the purpose of the study. The calorific power was measured in a calorimeter.
Methods
The wood samples of each species was extracted and milled into powder to be oven dried. The calorific power of each sample was measured in an adiabatic calorimeter where the ignition temperature was noted.
The 6300 Isoperibol Calorimeter System requires avail-ability of Oxygen, 99.5% purity, with CGA 540 connection, 2500 psig as maximum. Approximately 2L of tap water, with a total hardness of 85 ppm or less, are required for filling the calorimeter jacket reservoir. The inlet pressure was 30 psi. The required flow rate is on the order of 0.5 L/min. The temperature of the water should not exceed 25°C.
The Calorimeter automatically makes all the calculations necessary to produce a gross heat of combustion for the sample. Corrected temperature rises reading automatically, and the HHVh was determined, taking into the correction of fuse combustion by the following equation:
Where HHVh is the Higher Heating Value at constant volume of the fuel as analysed, in MJ/Kg; K1is a conversion factor (4.1855 .10-3KJ/cal; Ecalis a calorimetric equivalent of the Calorimeter apparatus in Cal/°C; Tm is the maximal temperature in °C; Tiis the minimal temperature in °C; L is the burned palatine fuse longer in cm; Ept is the Higher Heating Value at constant volume of the palatine, in MJ/Kg(=2.3 cal/cm) and M is the mass of the sample in g.
Precise temperature measurements are made with Thermistors thermometry providing 0.0001°C resolution over the operating range of the calorimeter. This system differs from adiabatic operation in which the jacket temperature must be adjusted continuously to match the bucket temperature in an attempt to maintain a zero temperature differential with no heat leaks between the bucket and its surroundings. Higher Heating Value in dry basis calculated by the equation according to CEN/TS 14918:13
Where HHV0 is the Higher Heating Value at constant volume of the dry (moisture-free) fuel, in MJ/Kg; Mad is the moisture in the analysis sample, in % by mass; HHVh is the Higher Heating Value at constant volume of the fuel as analysed, in MJ/Kg.
The Low Heating Value can be determined at constant pressure or at constant volume. The Low Heating Value at constant pressure is however the generally used, since it is the one that is usually used in combustion. His determination is fundamental at the time of evaluating a substance and also gives an idea of the potential to generate and propagate fires. The Low Heating Value at constant pressure for a dry sample is derived from the corresponding Higher Heating Value according to equation in CEN/TS 14918 [13]:
Where: LHV0 is the Low Heating Value in dry basis at constant pressure in MJ/kg;HHV0 the Higher Heating Value in dry basis in MJ/Kg; w(H)d is the hydrogen content, in % by mass, of the moisture-free (dry). Remark: (H), (O), (N) contents (% dry basis) are the value determined in the elemental composition analysis.
Low Heating Value (as received) calculated according to CEN/TS 14918:13
Where: LHVh is the Low Heating Value (at constant pressure) as received (MJ/Kg); LHV0 is the Low Heating Value (at constant pressure) in dry basis (MJ/kg); Mar is the moisture content as received [w%]; 0.02443 is the correction factor of the enthalpy of vaporization (constant pressure) for water (moisture) at 25°C [MJ/kg per 1 w% of moisture].
Where Mash is the mass of the ash (g) and oven-dry is the mass of oven-dried sample (g).
Calorific values
Figure 1 shows the values of the higher calorific value obtained for the different eucalyptus species. The examination of the high calorific capacity of the wood species shows that is ranged from 4017kcal/kg and 4541 kcal/kg with a maximum observed at Eucalyptus Stricklandii and a minimum observed at Eucalyptus patellaris. Figure 2 illustrates the values of the temperature of ignition and that of the hearth of the species of eucalyptus. The temperature of wood ignition of these species lies between a maximum observed for Eucalyptus Brockwayi (390°C) and one minimum observed for Eucalyptus largiforens (292°C). Moreover the figure shows the variation in temperature of the hearth. It has a maximum value observed at Eucalyptus brockwayi (575°C) and a minimal value observed at Eu. gillii (463°C).
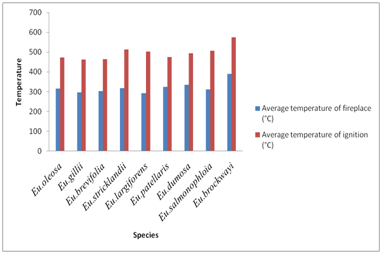
Figure 2 Ignition and the hearth temperatures of some species of Eucalyptus of Arboretum Hajeb Laayoun.
Ash content
Ash is formed from mineral matter during combustion and gasification. The ash yield of wood grown in the temperate zones is 0.1-1.0%, whereas wood grown in the tropics contains up to5% ash.15 The bark contains 3-8%, ash. Wood ash typically includes 40-70% calcium oxide and 10-30% potassium oxide.16 The mineral content of wood and bark is highly variable between and within species and can vary with soil and growth rate.17 Figure 3 illustrates the percentage of ash resulting from combustion. Indeed, this percentage of ash is ranged from a maximum observed at Eucalyptus patellaris (2.25%) and a minimum determined at Eucalyptus demise (1.05%). This inter-species variation of Ash content could be explained by the fact of hardwoods species (Eucalyptus) have a complex anatomical structure which is better able to keep a lot of mineral compounds during its growing than softwood species.
Gas measurements
For the gas exhaust Figure 4 shows that the complete combustion of the wood of different species exhausts almost similar CO and CO2 percentantages. The CO2 exhausted was maximum or Eu. dumosa (15.5%) and Eu. Patellosa (11.5%) while the values of the CO exhausted were almost the same.
Based on this study on the determination of the energy characteristics of 19 forest species, we can conclude that these species constitute a source of energy. The important obtained results show that the wood of these species has a high calorific value which is considerable and variable according to the species and the tree. This high calorific value lies between 4017kcal/kg and 4541 kcal/kg, respectively for Eucalyptus patellaris and Eucalyptus Stricklandii. The highest temperature of the ignition was recorded for Eucalyptus brockwayi (390°C) while the lowed for Eucalyptus largiforens (bicolor), (292°C). A simple classification of the studied species based on the temperature of the hearth, puts the Eucalyptus brockwayiat the top with a value of 575°C and Eucalyptus gillii at the bottom with a value of 463°C. The percentage of ash reached its maximum level with Eucalyptus patellaris with a value of 2.28 % and its minimum for Eucalyptus dumosa (1.53%).
None.
The author declares no conflict of interest.

©2017 Khouaja, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.