MOJ
eISSN: 2572-8520


Review Article Volume 4 Issue 1
1Department of Civil Engineering , Higher Institute of
Technological Studies of Sfax, , Tunisia
2National Engineering School of Tunis, University Tunis El
Manar, Tunisia
Correspondence: Malek Jedidi, Department of Civil Engineering, Higher Institute of Technological Studies of Sfax, B.P.88, 3099 Sfax, Tunis, Tunisia, Tel 216-982-586-81
Received: November 23, 2017 | Published: January 31, 2018
Citation: Jedidi M, Benjeddou O. Chemical causes of concrete degradation. MOJ Civil Eng. 2018;4(1):40-46. DOI: 10.15406/mojce.2018.04.00095
The durability of reinforced concrete structures depends on their behavior in relation to the climatic and environmental conditions that exist in the environments in which they are built. These structures are often subject to a permanent process of physical and chemical degradation as a result of external aggression. In this context, this paper presents the different chemical causes of concrete degradation such as carbonation, alkali-reaction and sulphate reactions. The diagnosis and repair techniques of the infected concrete have also been developed.
Keywords: chemical degradation, carbonation, alkali-reaction, sulphate reaction, diagnosis, repair
AGR, alkali-granulate reaction; ESR, either external sulfate reaction; ISR, internal sulfate reaction
A concrete structure must withstand over time the various attacks or stresses to which it is subjected, as well as various actions such as wind, rain, cold, heat, the environment while maintaining its aestheticism. It must satisfy, at a constant level, the needs of users during its service life. The notion of durability of a structure results in a set of technical specifications based on direct or indirect test methods, experience and recommendations for implementation, manufacture and maintenance. In fact, it is now possible to define sustainability objectives and to choose concrete characteristics precisely according to the aggressiveness of the environment in which the structure is located and to optimize its characteristics in order to adapt them to the duration desired service. The specifications concern the nature and minimum cement dosage, the minimum compactness, the maximum value of the Water/ Cement ratio, the minimum encapsulation of the reinforcements and the maximum chloride content in the concrete. The diagnosis of the state of conservation of a reinforced concrete structure makes it possible to characterize the origin and the extent of the possible disorders. In general, this diagnosis follows a detection of disorders, but it is also requested in various settings, such as renovation work or regular inspections. A prior inspection of the structure makes it possible to make assumptions about the possible causes of the disorders, and thus to specify the operations to be performed, taking into account the operation of this structure. The mechanical functioning of the different parts of a structure is a very important point to consider.
Indeed, if the phenomenon that causes deterioration of the base concrete is not controlled or if its diagnosis is incorrect, it is very likely that this mechanism of degradation also affects the repair concrete. An incorrect identification of the source of the problem then results in costly, unsuccessful and especially recurrent interventions, which is certainly not interesting from the point of view of the sustainability of the works and budgets, sometimes limited, managers. Concretes can be confronted with chemically aggressive environments that cause their degradation such as carbonation, alkali-reaction and sulphate reactions.1,2 The natural concrete carbonation depends simultaneously on the materials’ characteristics and the surrounding environment and it is associated with diffusion in concrete. It causes the reinforcement de passivation leaving the steel susceptible to corrosion.3–6 The most common repairs to concrete structures are either wet or dry process concrete, or formwork with or without concrete overlay, or more recently self-leveling concrete. This innovative technique is currently the subject of all intentions. This material is so fluid and lends itself to the most complex forms without being vibrated. The repair of a concrete element that usually involves two very different materials, where the placement of a young concrete on an older concrete support causes different types of problems, both physico-chemical and mechanical, related to the broad compatibility of the two materials in contact.
Carbonation
Definition: The carbonation is a chemical phenomenon linked to the emission of carbon dioxide into the atmosphere.7–10 It is pathology of reinforced concrete which, with the time, reaches layers more and more important. It degrades the reinforced concretes and is in particular responsible for the exposure of their steel frames. Carbon dioxide enters gaseous form in concrete. It causes a reaction, called carbonation, with interstitial water. The carbonation front advances progressively from the facing. It converts calcium hydroxide “hydrated lime”, which is a base, and which gives the water in the pores of concrete a high pH (between 12.5 and 13), carbonate . This carbonation front lowers the pH of the interstitial solution from to about 9.11 This degrades the passivation of the reinforcements. From a chemical point of view this reaction is as well:
13
Carbonation process
When curing concrete and after evaporation of water, the pores partially fill with air. The atmosphere currently contains about 0.33 ml of carbon dioxide ( ) per liter of air. The is then likely to diffuse through the gaseous phase of the cement. It can be seen that the cement is totally saturated with water and carbonates only on their boundary layer because of an immediate clogging of the pores by formation of calcite. Carbonation in concrete can be divided into three main stages:

Figure 1C Chemical reaction between dissolved.
Figure 1 Damage due to the reinforcement corrosion process.
Factor |
Influence |
W/C ratio (water/cement) |
The lower the ratio, the lower the carbonation rate. |
Type of cement |
Cement concretes with secondary constituents (slags, fly ash, |
Cement dosage |
An increase in dosage decreases the carbonation depth |
Cure |
A good cure reduces the rate of carbonation, because |
Humidity |
The carbonation rate is |
Temperature |
An increase in temperature increases the rate of carbonation. |
Table 1 Factors that influence the rate of carbonation of concrete
Diagnosis and treatment of carbonation
The purpose of a diagnostic is to find the cause of the degradation. Destructive and Non-destructive methods of investigation can be used for the control of certain characteristics of the concrete.12 The following tests can be performed (Figure 2):
The advance of concrete carbonation is influenced by the exposure conditions and environmental conditions.14–16 After developing an adequate diagnosis, the repair can begin. It is essential to refer to regulations and standards, and the technical specifications contained in the technical data. The perimeter of the surface to be repaired must have sharp edges for the cleanliness of the repair. Degraded concrete areas must be removed to find the sound surface of the concrete. A jackhammer is usually used. After removal of friable parts and clearance of reinforcement, brushing and scraping to remove rust can occur (Figure 3A). A rust inhibitor is possible and can be applied. In the event of a significant reduction in the section of the steel, it will be necessary to strengthen or replace. The minimum overlap thickness of the reinforcement shall be respected in all cases as this is an important factor for its protection. After the reinforcement treatment we can apply the repair concrete (Figure 3B). The type of concrete required is chosen carefully according to the desired repair character. The chosen repair product will be applied once the surfaces have been thoroughly cleaned. An application of the mortar in successive layers of 5 to 50 mm maximum must be carried out by compressing it strongly using a trowel (Figure 3C). It is important to properly tamp the mortar around the reinforcement bars to avoid air inclusions. At the end, a traditional float of the repaired parts was realized (Figure 3D). A final painting with concrete protection property against carbonation can also be applied.
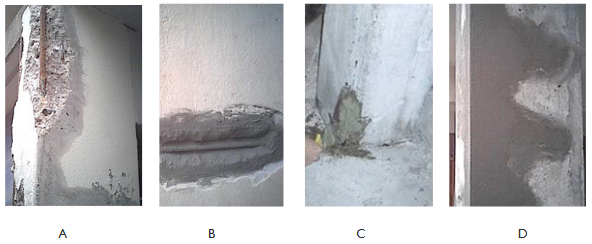
Figure 3 Treatment of carbonation: A: Treatment of reinforcement B: Repair concrete application C: Mortar application using a trowel D: Traditional float of repaired parts.
Alkali-reaction of the concrete
Definition: The alkali-reaction or the alkali-granulate reaction (AGR) is a chemical reaction between reactive aggregates and the alkalis contained in the cement.17,18 This reaction produces an expansion inside the concrete which will create tensions, then swelling and cracks (Figure 4). There are three types of reaction:
Prevention against alkali-reaction phenomena
The principle of the preventive approach consists in not being in a situation in which the three conditions necessary for the initiation of the reaction are simultaneously present. It is therefore necessary to avoid the conjunction of the three factors: water (relative humidity condition greater than 80-85%), amount of alkaline in the large concrete and reactive silica (presence of reactive aggregates). The prevention method is divided into two stages. It is based on the environment (Table 2) and the type of structure (Table 3) to determine the level of prevention to be achieved A, B or C (Table 4), and then verify that the formulation intended for concrete is satisfactory. It thus makes it possible to implement preventive recommendations adapted to the importance of the structure and its environment.
Classes |
Environment |
1 |
Dry or slightly humid (humidity below 80%) |
2 |
Humidity greater than 80% or in contact with water |
3 |
Hygrometry above 80% and with gel and flux |
4 |
Marine |
Table 2 Types of environment.19
Types of structures |
Risk level |
Examples of structures |
I |
Risks of occurrence of weak or acceptable disorders |
Non-carrying elements, Most precast concrete products |
II |
Risks of occurrence of intolerable disorders |
Non-carrying elements, Most civil engineering structures |
III |
Risks of occurrence of unacceptable disorders |
Tunnels, dams, bridges, viaducts |
Table 3 Types of structures.19
Types of structures |
Exhibition Classes |
|||
1 |
2 |
3 |
4 |
|
I |
A |
A |
A |
A |
II |
A |
B |
B |
B |
III |
C |
C |
C |
C |
Table 4 Risk level.19
The recommendations to be applied depend on the level of prevention
Level A: No specific specifications;
Level B: Six possibilities of acceptance of the concrete formula;
Level C: Non-reactive aggregates (PRP granulates under conditions).
Disorders due to the alkali-reaction
Figure 5 shows the alkali-reaction disorders that may occur generally at varying time intervals of two to ten years or more. The openings of the cracks are variable according to the progress of the reactions. They can be a few tenths of a millimeter for a small cracking and reach a few millimeters for cracking with large meshes. Crack openings can be measured using a fissurometer. The principle is to place the fissurometer over the crack and slide it until a graduated line exactly superimposes over the width of the crack (Figure 6).
Repair of disorders due to the alkali-reaction
There are some methods that can slow the evolution of reactions or stop the evolution of disorders such as:
Sulphate reactions
Definition: Sulphate attacks have a major problem of concrete durability. They destroy the concrete by degrading its mechanical properties. These include sulphate reactions that cause swelling in concrete and crack networks. The sulphate attack is associated with the precipitation of secondary sulphates, a significant expansion and the chemo-mechanical deterioration (changes in properties, cracks, loss of strength and cohesion). This can lead to the ruin of the cementitious material, more or less long term depending on the attack (nature, content and concentration of sulphates in contact) and the cement used (type and W/C ratio).
Types of sulphate reactions
Sulfate reactions are categorized into two broad classes based on the origin of source of sulfate (Figure 7), as either external sulfate reaction (ESR) or internal sulfate reaction (ISR).
Internal sulphate reactions ISR:
The internal sulphate reaction ISR phenomenon has been encountered in several countries in prefabricated concrete railway ties that have undergone heat treatment. These countries include Finland in 1987,20 Germany in 1989,21 Australia in 1992,22 the United States in 199523 and more recently Sweden in 2004.24 The RSI phenomenon has also been identified in some prefabricated concrete elements. In particular, this reaction has been demonstrated in a US parking staircase,25 in prestressed girders and gutters in the United Kingdom,26 in a stadium stand in the United States27 and in fiberglass roofs-cement in Italy.28 In the majority of these prefabricated elements, the disorders were observed in less than ten years after construction. The ISR is a pathology of cementitious materials related to the formation of a hydrate which is ettringite. Ettringite is a mineral species containing sulphates, It is a calcium trisulfoaluminate hydrated in cement notation resulting from the reaction between the calcium aluminates and the gypsum. An ettringite layer is then formed around the anhydrous cement grains. Figure 8 gives the three conditions that must be met to trigger the ISR. The ISR are characterized by surface cracks that appear after several years of exposure to severe conditions characterized by high humidity (Figure 9). This rare phenomenon can occur only in damp environments, in massive cast-in-place concrete parts in summer or on heat-treated concrete parts.
External sulphate reactions ESR
External sulfate reaction ESR is a chemical breakdown mechanism where sulfate ions from an external source attack components of the cement paste. It occurs when a cementitious material is in direct contact with a sulphate source, such as in soils, groundwater, seepage water, acid rain (sulfuric acid) related to industrial pollution atmospheric (Figure 10). The often massive formation of gypsum and ettringite formed during the ESR may cause concrete to crack and scale. However, both laboratory studies and examinations of field concrete show that external sulfate attack is often manifested, not by expansion or cracking, but by loss of cohesion and strength. The ESR essentially depends on the following parameters: The quality of the concrete, namely the composition of the cement, the method of manufacture, the cure, the state of damage of the concrete before the attack. The exposure on the site namely the concentration of and its distribution in the soil, moisture, transport opportunities.
Prevention of disorders related to ISR
The precautions to be implemented depend on a level of prevention defined for each part of a potentially “critical” structure. They take into account:
The recommendations concern only parts of large concrete structures in contact with water or in a humid environment. These are massive pieces or “critical” for which the heat released during the hydration of the cement (the setting and the hardening of the concrete generate a release of heat due to the exothermic reactions of hydration) is little evacuated to the outside, which leads to a significant rise in the temperature at the heart of the concrete. The principle of the preventive approach is to identify the parts of structures likely to be subjected to the phenomenon of ISR, then to define a level of prevention necessary according to the category of the work or the part of work and the exposure classes specific to the ISR, reflecting the environment in which the concrete is located. At each level of prevention (As, Bs, Cs, Ds) there is a level of caution to apply (Table 5). It is then necessary to implement for each part of work concerned the precautions adapted to each level of prevention.
Level of prevention |
Maximum temperature |
Limit temperature of |
Conditions to be respected if temperature |
AS |
85°C |
- |
- |
BS |
75°C |
85°C |
- Mastery of heat treatment or Suitable cement or - Performance test |
CS |
70°C |
80°C |
- Mastery of heat treatment or Suitable cement or - Performance test |
DS |
65°C |
75°C |
- Suitable cement; Validation of the formulation |
Table 5 Summary of the precautions to be applied to the ISR
The present paper has presented the different chemical causes of concrete degradation as well as the diagnosis and repair techniques of the infected concrete. The following conclusions have been drawn from the investigation:
None.
The author declares there is no conflict of interest.

©2018 Jedidi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.