MOJ
eISSN: 2574-819X


Mini Review Volume 1 Issue 1
1Department of Chemistry, University of Houston-Clear Lake, USA
2Research Service Division, WuXi AppTec (Wuhan) Company Ltd, China
Correspondence: Anton V Dubrovskiy, Department of Chemistry, University of Houston-Clear Lake Houston, TX, 7058, USA, Tel (281) 283-3769
Co-correspondence: FengShi, Research Service Division, WuXi AppTec (Wuhan) Company Ltd.666 Gaoxin Road, East Lake High-Tech Development Zone Wuhan 430075, P. R. China
Received: February 28, 2017 | Published: April 12, 2017
Citation: Dubrovskiy AV, Shi F. Transfer hydrogenation vs nucleophilic addition: aryne reaction with alcohols. MOJ Biorg Org Chem. 2017;1(1):9-11. DOI: 10.15406/mojboc.2017.01.00004
benzyne, nucleophilic addition, transfer hydrogenation
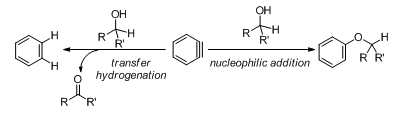
Aryne1 intermediates have been well recognized for their soft electrophilic nature that enables them to react with a wide variety of electron-rich substrates, often in a synthetically useful manner. A surprising exception to this reactivity is aliphatic alcohols which, despite their nucleophilic nature, are generally unreactive with arynes. Although several intermolecular examples of benzyne reacting with simple alcohols can be found in the early literature, most of such aryl ether-forming reactions afford products in marginal yields and rely on the use of alcohols as solvents.2-5 Recent advances where arynes are generated from the Kobayashi precursor (o-trimethylsilylaryl triflate)6 have only reiterated that stoichiometic quantities of aliphatic alcohols do not undergo nucleophilic addition to arynes in a productive manner.7 As an illustrative example, when carbodiimide, a not well recognized nucleophile, and methanol are both present, aryne would selectively react with the former 8. This seemingly counterintuitive “inertness” of benzyne toward aliphatic alcohols, along with some others highlighted in our recent review,9 constitutes a long-standing curiosity for aryne chemists, both synthetically and mechanistically.
This curiosity has now been addressed computationally by Hoye and co-workers (Scheme 1).10 The expected alcohol nucleophilic addition to aryne (Scheme 1, right) was found to be second-order with respect to the alcohol (Figure 1), and consequently is favored only at high concentration of the latter. This computational result is consistent with early success where alcohols were used as solvents, and is also consistent with Hoye’s and Lee’s earlier results where large excess of alcohols was typically used.11-12 Interestingly, this mechanistic rationale is in good agreement with a half-century-old finding from Bunnett that alcohol addition to arynes comes with accumulation of negative charge on aryne.4
Scheme 1 Computational mechanism for the second-order nucleophilic addition of MeOH to benzyne (DFT, M06-2X/6-311+G (d, p) level with SMD solvation model in MeOH. Units in kcal/mol).
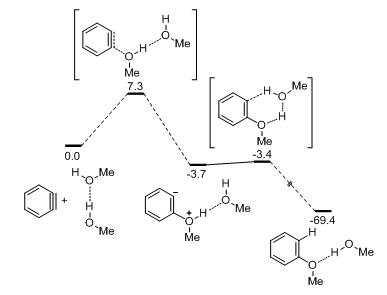
Figure 1 Computational mechanism for the second-order nucleophilic addition of MeOH to benzyne (DFT, M06-2X/6-311+G (d, p) level with SMD solvation model in MeOH. Units in kcal/mol).
Additionally, Hoye reported that at low alcohol concentration the reaction can take an alternate path, an Oppenauer- or Cannizzaro-like transfer hydrogenation reaction that oxidizes the alcohol into a carbonyl compound and reduces the aryne into an arene (Scheme 1, left). This novel mode of reactivity is first-order with respect to the alcohol and is calculated to proceed via a concerted, asynchronous mechanism (Figure 2). The aryne ring in the transition state is quite distorted13,14 and the H-C(OH) bond cleavage is more advanced than the cleavage of the O–H bond. Both findings support a minor contribution of a hydridic nature of the transferred hydrogen (H at the carbinol atom) within the concerted mechanism frame (a real carbocation mechanism is excluded). Due to its kinetic profile, the transfer hydrogenation is favored at low alcohol concentrations (Figure 3A). Supportive of the proposed mechanism, this process was also shown to be favored for substrates with weaker H–C(OH) bonds (Figure 3B).
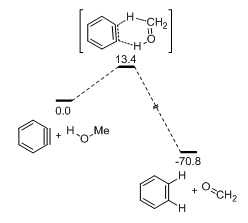
Figure 2 Computational mechanism for the concerted transfer hydrogenation of MeOH to benzyne (DFT, M06-2X/6-311+G(d,p) level in gas phase. Units in kcal/mol).
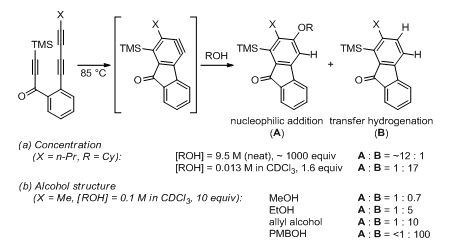
Figure 3 Nucleophilic addition vs transfer hydrogenation: dependence on the concentration and structure of alcohol (Cy = cyclohexyl, PMB = 4-methoxylbenzyl).
This previously unrecognized transfer hydrogenation and equally unrecognized second-order alcohol addition have collectively explained the poor reactivity in the addition of alcohols to arynes. Meanwhile, it also sparked another curiosity standing equally elusive, that is, whether the lack of reactivity of aliphatic carboxylic acids and primary carboxyamides with arynes7 could be explained by the same model. In fact, if one compares Hoye’s and Lee’s results11,12 where acetic acid underwent nucleophilic addition to arynes with Larock’s conditions7 where no synthetically meaningful yield was obtained, it follows that the former success can be, at least partially, attributed to the excess and higher concentration of AcOH15 which in turn might suggest higher-order kinetics with respect to the acid. It remains questionable as to why phenols behave differently from aliphatic alcohols in the nucleophilic addition with arynes, and why aromatic carboxylic acids behave differently from aliphatic ones.7
The transfer hydrogenation reactivity may also be more general than imagined. Hoyle reported that other than alcohols, medium-sized cycloalkanes and even THF are good hydrogen donors to reduce arynes.16 Okuma also reported17 that a‑amino acid methyl esters derived from phenylalanine and aspartic acid resulted in significant dehydrogenation (product E, Figure 4) after annulation to indolin-3-ones. Because tyrosine counterpart did not afford the dehydrogenation product, this process is unlikely aerobic. Therefore, a transfer hydrogenation (Figure 4) is possibly in operation. This and related dehydrogenation cases need to be addressed in the future work.
In summary, the work of Hoye and co-workers shed light on previously unexplained reactivity of arynes with alcohols. Well supported by experimental evidence, this computational study not only explains the apparent inertness of alcohols toward arynes, but also provides solid evidence for a novel transfer hydrogenation process that can be in prospect applied to a broader scope of aryne-participated redox schemes. It is a good demonstration of how diligent studies of seemingly well understood reagents/reactions can unfold new and unexpected modes of reactivity.
This work was partially supported by the Robert Welch Foundation (departmental grant BC-0022).
The author declares no conflict of interest.

©2017 Dubrovskiy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.