MOJ
eISSN: 2574-819X


Research Article Volume 1 Issue 4
Department of Studies in Chemistry, Mangalore University, India
Correspondence: Boja Poojary, Department of Studies in Chemistry, Mangalore University, Mangalagangothri, Karnataka, 574199, India, Tel +91-824-2287262, Fax +91-824-2287367
Received: August 07, 2017 | Published: August 29, 2017
Citation: Bhat M, Poojary B. One-pot synthesis of a new imidazole-5-carboxylic acid derivative via heterocyclization reaction. MOJ Biorg Org Chem. 2017;1(4):113-116. DOI: 10.15406/mojboc.2017.01.00020
In the current study, a new benzimidazole molecule 2-(3-bromo-4-hydroxy-5-methoxyphenyl)-1-methyl-1H-benzo[d]imidazole-5-carboxylic acid was synthesized via the hetero cyclization of ethyl 4-(methylamino)-3-nitrobenzoate with 5-bromo-4-hydroxy-3-methoxybenzaldehyde in the presence of sodium dithionite in DMSO followed by the base hydrolysis. The synthesised compound was characterized using IR, NMR (1H and 13C) and Mass techniques.
Keywords: heterocyclization, benzimidazole, Na2S2O4
DMSO, dimethyl sulfoxide; Na2S2O4, sodium dithionite; TMS, tetramethylsilane; EtOH, ethanol; NaOH, sodium hydroxide
From few decades, fused heterocyclic chemistry has occupied a prominent place in Medicinal Chemistry. Moreover, fused imidazole systems have attracted the researcher’s interest due to their natural occurrence and significant medicinal applications.1-3 Additionally, the benzimidazole derivatives are the bioisosters of natural nucleotides, which can interact easily with the macromolecules (proteins, enzymes and receptors) of the biological system, has made the benzimidazole and its derivatives as the privileged pharmacophore in drug discovery process.4,5 They are able to produce anti-inflammatory,6 antibacterial,7 anti cancer,8 analgesic,9 antiviral10 and anticonvulsant11 activitie, etc., Further benzimidazoles also can act as inhibitors for α-Glucosidase,12 Cyclooxygenase,13 Poly (ADP-ribose) polymerase14 and lysophosphatidic acid acyltransferase-β.15 Interestingly, benzimidazoles are also being incorporated into various commercially available drugs.16,17 This vast applicability and usefulness of the fused imidazole nucleus inspired us to design and develop the target molecule.
Further, harsh and vigorous reaction conditions as well as tedious work up procedures18,20 of the classical methods which are available so far for the synthesis of benzimidazole derivatives necessitate the simple one-pot reductive cyclization method. Furthermore, several new one-pot methods which were reported in recent years utilises either the costly reagents or hazardous reaction conditions.21-23,27 Moreover, Sodium dithionite (Na2S2O4) a protean reducing agent was able to reduce several functional groups like aldehydes, ketones, conjugated ketones and nitro group. It was also been reported as an agent during one-pot synthesis of the benzimidazole and its derivatives.24-26 Thus, advantage of one-pot synthesis to conduct the reaction in more sustainable way by minimizing the use of reagents and solvents as well as with the reduction in reaction time has motivated us to utilize the same in our research.
Synthetic strategy for the title compound is as depicted in the Figure 1. A new benzimidazole derivative (2) was synthesized by reacting ethyl 4-(methylamino)-3-nitrobenzoate (1) with 3-bromo-4-hydroxy-5-methoxybenzaldehyde in the presence of three equivalence of Na2S2O4 in DMSO at 90°C.18 Base hydrolysis of the compound 2 yielded the title compound (3). The synthesis of ethyl 4-(methylamino)-3-nitrobenzoate (1) was explained in our earlier report.19
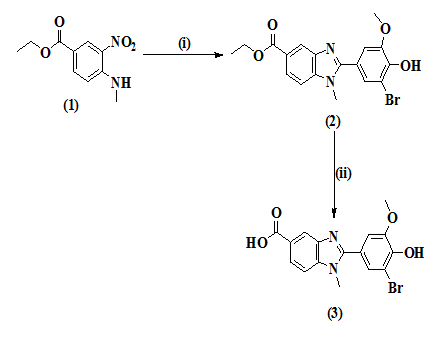
Figure 1 Synthesis of 2-(3-bromo-4-hydroxy-5-methoxyphenyl)-1-methyl-1H-benzo[d]imidazole-5-carboxylic acid. Reagents: (i) 3-bromo-4-hydroxy-5-methoxybenzaldehyde, Na2S2O4, DMSO, stir, reflux at 90 ᵒC (ii) EtOH, NaOH (33%), and reflux.
Formation of the target molecule was confirmed by the IR (ATR) data. A broad band near 3394 cm-1 justified the presence of -OH group. A characteristic stretching for acid carbonyl was observed at 1701 cm-1. Further, the stretching frequencies for the aromatic and aliphatic hydrogen were observed at 3065, 2906 and 2870 cm-1, respectively. An absorption band at 1624 cm-1 is corresponding to the functional group C=N and a sharp band near 525 cm-1 confirms the presence of a bromine atom in the molecule (Supplementary Figure 1).
The 1H NMR spectrum also evidenced the formation of the title compound. A characteristic singlet at δ 3.88 ppm was assigned to the three protons of the methoxy group. A peak at δ 3.91 ppm is ascribed to the –N-methyl protons. Additionally, the spectrum exhibited a doublet for C2-H of 3-Br-4-OH-5-OMe-C6H2 at δ 7.40 ppm (J = 1.6 Hz) and a proton on the 6th carbon of the 3-Br-4-OH-5-OMe-C6H2 ring exhibited a doublet at δ 7.56 ppm with coupling constant 1.6 Hz, due to meta coupling. The protons on the 7th and 4th carbon atom of the benzimidazole ring displayed two doublets at δ 7.66 (J = 8.2 Hz) and 8.21 ppm (J = 1.2 Hz) correspondingly. A doublet of doublet at δ 7.89 ppm (J = 8.8 and 1.6 Hz) was accredited to the proton on the 6th carbon atom of the benzimidazole ring. A characteristic broad singlet was appeared at δ 10.12 ppm for the hydroxyl group on the 4th position of the 3-Br-4-OH-5-OMe-C6H2 ring. An acid proton has resonated to give a broad singlet at δ 12.79 ppm (Supplementary Figure 2).
The target molecule is also confirmed by its 13C NMR, which has given a characteristic carbonyl signal at δ 168.3 ppm. Further, spectrum also exhibited signal at δ 154.4 ppm, which corresponds to -C=N- of the benzimidazole ring. The 4th carbon of the 3-Br-4-OH-5-OMe-C6H2 ring resonated at δ 148.7 ppm and the 5th carbon of the same, which is having methoxy substitution, was assigned at δ 146.1 ppm. The signals at δ 142.2 and 140.1 ppm corresponds to the two =C-N- carbon of the benzimidazole ring. The peaks at δ 125.8, 125.1, 124.0, 121.8, 121.1, 112.7, 111.8, 109.7 ppm was assigned for the remaining aromatic carbons. A methoxy and -N-CH3 carbons were resonated at δ 56.9 and 32.4 ppm, respectively. Molecular weight of the compound was in agreement with the data obtained from ESI-MS spectrum with peaks at (m/z) 376.95 (M+ + H), 378.90 [(M+ + H) + 2]. Melting point of the compound was 260°C (Supplementary Figure 3).
Laboratory grade chemicals were procured and desired molecules were synthesized with the aid of standard techniques. Melting point of the target molecule was determined using the open capillary method. The novel synthesized molecules were established and characterized with the help of Thin-layer chromatography (TLC), FTIR spectra were recorded on a Shimadzu ATR Spectrometer, 1H and 13C NMR data from Bruker DRX-300 and 400 MHz NMR spectrometer and Bruker DRX-75 and 100 MHz NMR) in (DMSO)-d6, respectively (Tetramethylsilane (TMS-an internal standard)) and ESI-MS spectra by Shimadzu LCMS 2010 spectrometer (Supplementary Figure 4).
Synthesis of the title compound
A reaction mixture containing ethyl 4-(methylamino)-3-nitrobenzoate (1) (0.006 mol), 3-bromo-4-hydroxy-5-methoxybenzaldehyde (0.006 mol), sodium dithionate (0.024 mol) in DMSO was refluxed with stirring at 90ᵒC for 3 h. The completion of reaction was monitored by Thin Layer Chromatography (TLC). After completion, the reaction mass was cool to room temperature and poured on crushed ice. The solid formed was collected, filtered and dried, which afforded the compound (2). The hydrolysis of compound (2) with 15 mL (33%) NaOH in ethanol yielded the required molecule (3) (Figure 2).
In this present research, a novel benzimidazole acid was easily synthesized with the help of “one-pot” method, followed by the base hydrolysis. The compound under interest was characterized and confirmed with the help of analytical and spectral techniques.
The authors are thankful to the director, Mysore University for providing the NMR data. Authors are also thankful to Mangalore University for rendering the facilities for the research.
All the authors declare that they have no Conflict of interest.

©2017 Bhat, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.