MOJ
eISSN: 2574-819X


Research Article Volume 1 Issue 6
1Universidad Juarez del Estado de Durango, Mexico
2Departamento de Ciencia y Tecnologia de los Alimentos, University in Reynosa, México
3Instituto Tecnologico de Tepic, Mexico
Correspondence: Erick Sierra-Campos, Facultad de Ciencias Químicas, Universidad Juarez del Estado de Durango. Av. Articulo 123 S/N Fracc. Filadelfia. CP 35010. Gomez Palacio, Durango, Mexico, Tel (+52) 8717158810, Fax (+52) 8717158810
Co-correspondence: Miguel Aguilera-Ortiz, Facultad de Ciencias Químicas, Universidad Juárez del Estado de Durango. Av. Artículo 123 S/N Fracc. Filadelfia. CP 35010. Gómez Palacio, Durango, México
Received: November 28, 2017 | Published: December 13, 2017
Citation: Lira de la Mora JA, Sierra-Campos E, Sánchez-Muñoz MA, et al. Effect of high hydrostatic pressures on antioxidant properties of mexican fig ( ficus carica l .) paste. MOJ Biorg Org Chem. 2017;1(6):234-237. DOI: 10.15406/mojboc.2017.01.00040
The fig is a fruit with important nutritional and functional properties for human health. The aim of this study was to evaluate the impact of processing with high hydrostatic pressures (HPP) at different times and temperatures of pressurization on anthocyanin content and antioxidant capacity of fig paste. This work showed that HPP at 350MPa for 5, 10 and 20min at 20 and 40°C did not change total acidity, total soluble solids and color. However, pressurized samples at 350MPa for 5, 10 and 20min showed a significant increase in anthocyanin content compared to the control. The antioxidant capacity, measured by 2,2-azino-bis-3-ethylbenzatholine-6-sulfonic acid assay was not altered by HPP at different times, but when it was treated at 350MPa by 20 and 40°C, this result was modified and had a significant increase when method of 2,2-diphenyl-1-picrilhydrazil was used. These data demonstrate that the HPP can be used in the fig products generation with higher nutritional quality.
Keywords: fig paste, HHP, antioxidant capacity, anthocyanin, PPO
Common fig (Ficus carica L.) belongs to the family Moracea. It is commonly known as fig, which is a medium-sized deciduous tree widely distributed in sub-tropical and tropical countries. The fig was introduced in Mexico by Spanish Franciscan missionaries in the 16th century and it is assumed that Mexican figs are the Spanish cultivar Black Mission.1 This fruit has been used as medicine for several centuries,2 hence being an important harvest worldwide for its dry and fresh consumption. Moreover, fruits can be eaten canned, or in other preserved forms.3 However, these procedures may alter the phytochemical composition of the product.
Nowadays, there is a general trend to increase fresh fruit consumption mainly due to their health properties. Different studies have demonstrated that figs are an important source of minerals and vitamins such as iron, calcium, potassium, thiamin and riboflavin.4 Figs are free of sodium, fat and cholesterol; contain at least 17 amino acids and high concentrations of aspartic and glutamic acid.5,6 They also possess relatively high fiber content (5.8%, w/w), which more than 28% is soluble, and can help to lose weight and to control blood sugar and cholesterol.7 Dehydrated figs have the highest concentration of polyphenols when compared to regular fruits and generally consumed beverages.5 For all these characteristics, figs are really considered as functional foods.8
Thermal processing is traditionally used in food industries causing unwanted changes in food quality such as loss of aroma, color, flavor, texture and nutritional value. The use of high hydrostatic pressures (HHP) for food processing has increased its application by food industry. HHP is a non-thermal technology capable to produce high quality food, preserving the characteristics of fresh food and extending its shelf life. In addition, HHP have the ability to inactive microorganisms as well as enzymes responsible for shortening the life product while maintaining food sensorial and nutritional properties.9,10 However, in some cases, HHP may activate undesirable enzymes such as polyphenoloxidase (PPO) resulting in short shelf life due to flavor and color changes.11
Phenolic compounds are closely associated with the sensory and nutritional quality of fresh and processed foods. Among the chemical reactions that occur during fruits processing, phenolic compounds oxidation is a phenomena responsible for profound modifications of the initial plant polyphenols concentration. Since it is generally agreed that PPO is mainly responsible for browning, and its increased activity after peeling and cutting would be expected.12
Due fig is a climacteric fruit, very perishable, with high metabolic activity, fast ripening and reduced store time at room temperature, make difficult its commercialization. Furthermore, the industry of fresh-cut fruits and vegetables is constantly growing due to the current consumption needs for minimally processed foods. Therefore, new techniques for maintaining quality and inhibiting undesired microbial growth are demanded in all steps of the production and distribution chain.13 Thus, the application of HPP seems to be a good processing alternative for fig. The main aim of this work was to evaluate the effect of HPP on physicochemical properties, anthocyanin content and antioxidant capacity in fig paste (Black mission).
Reagents
ABTS reagent (2,2-azino-bis-3-ethylbenzthia-6-sulfonic acid), potassium persulfate and Trolox was purchased from Sigma-Aldrich. Anhydrous ethanol, anhydrous methanol, HCl and all other reagents were of analytical grade.
Sample preparation
Figs (Ficus carica L.), Black mission variety, were freshly cut and received from Huerto La Linda, S.P.R de R.L. of C.V. located in the Ejido Álvaro Obregón from Lerdo Durango, Mexico. Figs were washed with fresh water and stored in a cooler with crushed ice, in a proportion less than 20% of the volume of fig to slow maturation without causing cold damage, until its use (less than 6 hours). Peduncle from fruit was removed with a knife and figs were liquefied without adding water in an industrial blender of 4 Kg (International model LI-5 with serial number 037-ago-98). Liquefied samples were placed into a 60 L aluminum container where the whole fig batch was mixed and stored under refrigeration until processing (less than 12 hours). The homogenized fig paste was weighed (100 g) inside vacuum packing bags, impervious to oxygen and moisture, and vacuum sealed with a sealer (Food Saver® model U3835 Bag Sealer). Bags were labeled and subjected to pressurization. Paste was always in contact with ice to prevent the growth of microorganisms. The samples already packed in vacuum were placed in warm water to reach the pressurization chamber temperature.
Fig paste processing with high pressures
Treatments were carried out in an isostatic press (Laboratory Cold Isostatic Press model LCIP402260NCEP1MLN) located in the Integral Food Laboratory of the Technological Institute of Tepic (Nayarit, Mexico) with a maximum pressure of 700 MPa. The pressure transmitted to the fluid was distilled water containing 5% (v/v) of ethylene glycol, according to the manufacturer's instructions. The time required to reach the desired pressure was independent of the pressure level (350 MPa/ 4.18-5.29 min) and the time of pressure relief was 15-43 seconds. The pressurization times reported in this study do not include an increase in pressure. Black mission samples were pressurized at 350 MPa for 5, 10 and 20 min at 20 and 40°C. The pressurized samples at 0 min, means to pressurize enough time to reach the pressure of 350 MPa and at that time the pressurization is suspended. Control samples reached room temperature (20 or 40°C) and were cooled again. After processing, samples were stored at -20°C until its analysis. The experiments were developed using homogenized samples of each of two fig varieties and analyzed by triplicate.
Extraction and quantification of anthocyanins
To determine the total anthocyanin content, approximately 1 g of fig paste sample was weighed in a 25 mL Erlenmeyer flask and a solution to extract anthocyanins (10 mL) was added (HCl: methanol: water: 0.02:8:1.8; v/v/v). The flask was placed in a sonicator at 50°C for 1 h. The extract was centrifuged at 3000 rpm for 10 min at 4°C and supernatant was collected and stored in an amber vial at 4°C until its use. Finally, absorbance was measured at a wavelength of 525 nm and total anthocyanins concentration in fig paste was reported as μg equivalent of cyanidin 3-glucoside/g of fig paste.
Total antioxidant capacity
Antioxidant capacity was evaluated using the ABTS method according to Nenadis et al.14 A 7mM aqueous solution of ABTS and a 140 mM solution of potassium persulfate were prepared. Then, 88 μL of potassium persulfate were mixed with 5 mL of ABTS and left to rest in the dark for 12 h. Then, 500 μL of the activated radical was taken and mixed with 25 mL of ethanol. The absorbance was determined at 734 nm and adjusted from 0.7 to 1 if necessary. The calibration curve of Trolox was used in a range of 0.5-8 μΜ from a methanolic solution of Trolox sonicated for 5 minutes.
The inhibition percentage of DPPH was calculated as follows:
DPPH inhibition (%) = [(A ctrl – A) / A ctrl]* 100
Results are expressed as antioxidant activity equivalent to Trolox (μM Trolox/100 g of paste).
For DDPH determinations of fig paste samples, a plate containing the calibration curve and samples was read at 520 nm in an Elisa reader by following kinetic times of 0, 4, 10, 30, 60 and 90 min until the percentage of inhibition of the antioxidant (Trolox) was observed. Different Trolox alcoholic solutions were used for calibration curves and the analyses were performed in triplicate and results were expressed as DPPH inhibition percentage.
Statistical analysis
Data were analyzed by Statistica for Windows version 7.0 applying two-way analysis of variance and differences between means were determined (P< 0.05) using Duncan´s multiple range test.
Diverse studies on change in the state of food products have shown the impact of the quality of processing methods on the organoleptic properties (e.g. taste, texture, smell and appearance). The color is an intrinsic property of foods, and therefore, a change in color is often caused by change in quality. While other important quality attribute of processing figs is titratable acidity because the presence of citric, acetic and a small quantity of malic acids has been reported in figs and the level of acid contributes markedly to the flavor of the products.15 This study showed that HPP at 350 MPa for 0, 5, 10 and 20 min at 20 and 40°C did not change total acidity, total soluble solids and color (data not shown).
Figs are an important source of anthocyanins. In this study, the total anthocyanins content of fig paste at different pressurized-times are summarized in Table 1. Their values range from 237.3 to 271 µg/g of paste. Thus, the total anthocyanins content was significantly increased with increasing pressurized time (P<0.05). The fig paste at 10 min of pressurized time, showed the highest total anthocyanins when compared to other treatments. In this study, the content of anthocyanins did not change significantly in all the samples treated at different temperatures (20 and 40°C) at 350 MPa. However, pressurized samples at 350 MPa at different times (0, 5, 10 and 20 min) showed a significant increase in anthocyanin content compared to the control. In these samples, the contents were significantly higher (5-14%) and may be the result of cell disruption caused by pressure and leading to a higher extractability of these compounds.16
|
Time (min) |
Anthocyanins Content |
|
0 |
233.59b |
|
5 |
251.68ab |
|
10 |
271.08a |
|
20 |
266.20a |
|
Control |
237.31b |
Table 1 Anthocyanins content in pressurized and non-pressurized fig paste
Mean with different letter are significative different, p= 0.029
The effect of HHP on anthocyanins content has been well studied. When products rich in polyphenols and anthocyanins are pressurized, anthocyanins content is affected and increased with processing conditions (temperature and time).17 These results are agreed with Corrales et al.18 who have demonstrated the increase in anthocyanins recovery when HPP are applied. However, lower anthocyanin content was observed in samples treated for 20 min and it may be due to a remaining activity of enzymes such as PPO and β-glucosidase, due PPO is a stable enzyme to pressure, which oxidizes polyphenols (anthocyanins) generating dark compounds. In addition, García et al.19 observed the activation of this enzyme in red raspberries treated at 600 MPa for 10 min. However, PPO and other peroxidases are inactivated by applying a pressure equal or greater than 400 MPa in combination with temperatures between 20 to 90°C. Under these conditions, PPO activity can be reduced by up to 50%, although the percentages may vary depending on intrinsic properties of processed foods.20
Generally, temperature is one of the most important factors affecting antioxidant activity. The antioxidant capacity of a substance is reflected by its ability to scavenge reactive oxygen species and reactive electrophiles. The antioxidant capacity of the fig paste was 376.7 μmol Trolox/100 g of paste by ABTS. The antioxidant capacity of our treatments decreased when temperature is increased (Figure 1). HPP had a light negative effect on the antioxidant capacity measured by ABTS, reducing the antioxidant capacity by 21.5% when the pressurization temperature was raised from 20 to 40°C. As shown in Figure 1A, from 0 to 5 min of pressurization at 40°C, antioxidant capacity was increased, but from 10 to 20 min a slight decrease in antioxidant activity was observed. The decrease in antioxidant capacity can be the result of eliminating part of enzymatic oxidation, responsible for polyphenols and anthocyanins loss.
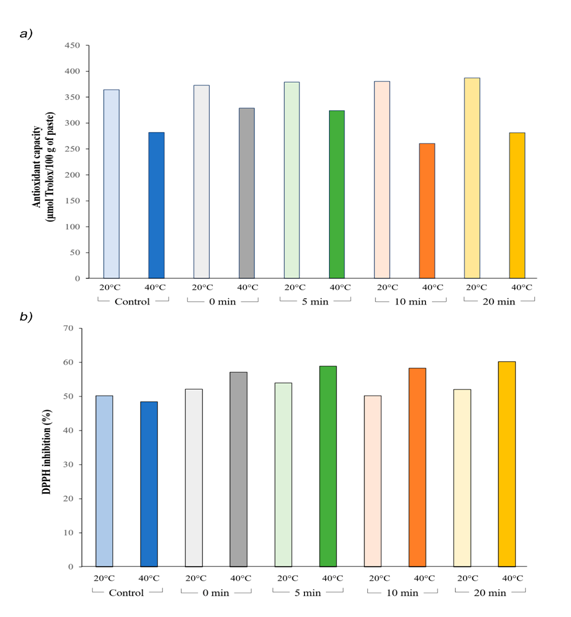
Figure 1 Effect of different temperature and time of pressurization on antioxidant properties of fig paste.
A) Antioxidant capacity with ABTS
B) DPPH radical scavenging activity of pressurized and non-pressurized fig paste. Data were expressed as mean. Values are significative different at P<0.05.
A number of methods are used to determine the radical scavenging effects of natural compounds with antioxidant properties. DPPH method is a preferred procedure due it is fast, easy and reliable and does not require a special reaction or device. When the stable DPPH radical accepts an electron from the antioxidant, a violet color of the DPPH is reduced to yellow which is measured spectrophotometrically. All treatments showed same level of DDPH radical scavenging activity, about 49.3% of antiradical activity by inhibition of DPPH. HHP treatment increased antioxidant activity in fig paste, which ranged from 50 to 60%. Thus, the DPPH method showed an increase of 14.4% of the antioxidant capacity after 5 min at 350 MPa (Figure 1B) and a 9.4% raise when the pressurization temperature ranges from 20 to 40°C.
It has been shown that the effect of HHP on antioxidant capacity depends on the treated product. For instance, Patras et al.21 reported higher antioxidant capacity of blackberry puree after pressurization, but found no effect of the process on strawberry puree. Tomato and carrot purees subjected to HHP presented higher antioxidant capacity than unprocessed samples.22 Cao et al.23 studied the total phenolic levels of strawberry pulps submitted to HHP, at 400 MPa there was a decrease in total phenolic content regardless the time of HHP treatment.
Time and temperature of HHP treatment showed the strongest influence on anthocyanin content and antioxidant activity. Thus, our results suggest that 10 min of HPP at 20°C would be appropriate operating conditions to produce favored anthocyanin levels, antioxidant capacity and scavenging activity in fig paste.
This research was partially supported by Programa para el Desarrollo Profesional Docente (PRODEP grant UJED-2016, SES-México).
The authors declare that there is not a conflict of interests regarding the publication of this paper.

©2017 Lira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.