MOJ
eISSN: 2574-819X


Research Article Volume 2 Issue 4
1Department of Biomedical and Genetics Hanoi Medical University Hospital, Vietnam
2Vietnam Military Medical University, Vietnam
3Hanoi Amsterdam High School for Gifted Students, Vietnam
4HUS High School for Gifted Students, Vietnam
Correspondence: Nguyen Thi Trang, Department of Biomedical and Genetics, Hanoi Medical University, N.1, Ton That Tung, Dong Da, Hanoi, Vietnam, Tel +8416 3878 8736
Received: July 06, 2018 | Published: August 3, 2018
Citation: Trang NT, Sang TT, Hoang N, et al. Assessment of the level of seminal zinc and fructose concentration in seminal plasma of Vietnamese infertile men. MOJ Biorg Org Chem. 2018;2(4):185-190. DOI: 10.15406/mojboc.2018.02.00079
This study assessed the association between the fructose and zinc concentration and various seminal characteristics. Fructose and zinc in semen reflects the secretary function of seminal vesicles. These Tests may help in assessing the diagnosis and the management of male infertility. Gather seminal plasma of 180males with average age of 31.1±3,6years old. Use the specific complex ant to form a stable colored complex with fructose or zinc. The colour intensity in a determining wavelength is proportional to the amount of fructose or zinc present in the sample. Seminal fructose concentration was significantly lower in oligozoospermic group and the azoospermic group in comparison with normozoospermic group. There are also many significant differences in Zinc’s concentration in semen when compared two in term of three groups, consist of the oligospermic, azospermic and normospermic group. Sperm DNA fragmentation index in-group with normozospermia correlated positively with seminal fructose (r=0.47, P<0.05) and negatively with seminal zinc (r=-0.56, P<0.05) concentrations. In group with oligozospermia found negative correlation (r=-0.24, P<0.05) between seminal fructose with sperm DNA fragmentation. In addition, there were 3 patients with Klinefelter syndrome in group with non-obtructive azoospermia with elevated seminal fructose concentration.
The role of seminal fructose concentration does not only lie in the assessment of seminal vesicle dysfunction but also in conjunction with other seminal properties could give a useful indication of male reproductive function whilst seminal zinc concentration might not be most appropriate for the assessment of male reproductive dysfunction. This is the first study to demonstrate that the increase of seminal fructose and decrease of zinc concentrations may reflected by high sperm DNA damage. This is the first report of increased in seminal fructose concentration in seminal plasma from the azoospermic men with Klinefelter's syndrome.
Keywords: infertility, seminal fructose, seminal zinc, azoospermia
CBAVD, congenital bilateral absence of the vas deferens; PESA, percutaneous epididymal sperm aspiration; DFI, DNA Fragmentation Index; HCl, hydrochloric acid; SPSS, statistical package for the social sciences
Infertility is defined as the failure of a couple to achieve a pregnancy after at least one year of frequent unprotected intercourse.1,2 It has been reported that the male partner contributes in 40% of the cases of infertility. Globally, the incidence of fertility is estimated to be about 13-18%.1,3 Due to the various reasons caused male infertility, it is essential to identify appropriate diagnosis methods to detect them. There are many tests that have been applied for several decades such as semen analysis, genetic tests and hormones methods. Recently, some of biochemical markers including zinc and fructose are becoming significant implications for diagnosing the cause in male infertility.4 They have thus been established as good indicators of human male fertility. An understanding of the factors affecting these characteristics is critical to proper understanding of the mechanisms underlying male infertility.5,6
Fructose is essential for spermatozoa metabolism and spermatozoa motility.7 Fructose is an energy source for spermatozoa. It is produced by the seminal vesicles with some contribution from the ampulla of the ductus deferens.8,9 Determination of seminal fructose concentration has been used in examination of obstructive azoospermia and inflammation of male accessory glands.10,11 The role of fructose concentrations in seminal plasma for total and sperm density has been investigated by several authors. Rajalakhshmi M, et al.,12 and Gonzales13 reported that an increase in sperm concentration is often accompanied by a decrease in fructose concentration in seminal plasma, because sperm using fructose as the primary source of energy,12,13 However, others studies have also shown that fructose concentrations in seminal plasma of patients with oligozoospermia and azoospermia did not decrease as compared to normal men.
Apart from fructose, zinc is another factor essential for male reproductive system. Deficiency of zinc in the reproductive system causes hypogonadism and gonadal hypofunction.14,15 Many studies have shown that zinc plays an important role in the normal development of testicles, prostate and sperm motility.9,16 However, in Vietnam, the knowledge about relationship between seminal zinc and fructose concentration on human sperm characteristic is insufficient and scanty. Therefore, the purpose of this study was to determine the association between the fructose and zinc concentration and various seminal characteristics in men. From there, we recommend determining the concentration of fructose and zinc for male infertility diagnosis with abnormalities on semen analysis.
WHO 2010 divided male infertility into 3groups: normozoospermia (sperm concentration >=15 billion/ml), oligozoospermia (sperm concentration<15 billion/ml) and azoospermia (no sperm).16 There are many studies determined seminal fructose and its correlations with concentration, vitality and motility of sperm. Azoospermia is the reason in 20% infertility man17 and the common reason of azoospermia is CBAVD.12,18
Study design was descriptive research. Fructose and Zinc concentration in seminal plasma of 180 patients with average age of 31.1±3,6years old. visited in Fertility Department of Hanoi Medical University Hospital after accomplished semen analysis with abnormalities sperm characteristic (sperm concentration, total count, motility, progressive motility) from March, 2016 to March, 2017, were used for study. All the samples were performed according to the World Health Organization criteria (1992). On the basis of the assessed parameters, sperm concentration and sperm motility were considered as the important parameters.
Measuring the concentration of fructose and zinc
The participants of the study were asked to produce sperm by masturbation and collected in sterile container with period of 2-5days of intercourse abstinence. Sperm should be analyzed within 2hours after produced. Routine semen analysis was performed according to WHO 2010 guidelines. After the semen analysis, samples were centrifuged at 1500 x g for 10min and zinc and fructose concentrations assayed from the supernatant (i.e. seminal plasma). Zinc concentration was assessed using spectrophotometry (5- Br- PAPS method) - direct colorimetric test without deproteinization of the sample. At pH 8.6, in a buffered media, zinc reacts with specific complexant 5-Br-PAPS form a stable color compound. Fructose content in seminal plasma was determined by the resorcinol method where fructose reacts with resorcinol in concentrated hydrochloric acid (HCl) solution to form a red compound. Measure the coloric complex of Zinc and Fructose at a wavelength of 560nm against blanks (ROE, 1976).19 Semen samples were collected for routine semen analysis and sperma DNA Fragmentation Index (DFI) testing. Technique of sperm DNA fragmentation test using Halosperm kit (Halotech Madrid, Spain).
Statistical analysis
Statistical analysis was performed using SPSS version 16.0. The means were compared using student t test. The statistical tests were considered to be significant at the p≤0.05 level.
Ethical considerations
Ethical approval to conduct the study was sought from the Hanoi Medical University. Permission to use data from the Hanoi Medical University Hospital was sought from the hospital authority. All the information from the database was kept under strict confidentiality. No names were recorded.
Table 1 shows that seminal fructose in oligozoospermia was significantly higher than normozoospermia (p<0.05). Besides, the mean sperm concentration (133.808±48.215 millions/mL), and the mean vitality (86.483 ±3.218%) and the mean progressive motility (11.250±10.157%) in males with normozoospermia were significantly higher than that in males with oligozoospermia (5.633±4.992 millions/mL and 58.183±18.14% and 11.250±10.157% respectively) (p<0.01).
Group |
Normozoospermia (n =60) |
Oligozoospermia (n =60) |
P |
Fructose (g/L) |
1.601±0,604 |
1.881±0,640 |
< 0.05 |
Sperm DFI (%) |
30.84±17,54 |
21.09±11,18 |
<0.01 |
Sperm concentration (billion/ml) |
133.808±48,215 |
5.633±4,992 |
< 0.001 |
Vitality (%) |
86.483±3,218 |
58.183±18,114 |
< 0.001 |
Progressive motility (%) |
54.667±9,278 |
11.250±10,157 |
< 0.001 |
Table 1 Seminal fructose and some characteristics of the semen
Some sperm characteristic and seminal fructose concentration were demonstrated in charts. The results show the correlation is significant at the 0.05 level (2-tailed) between seminal fructose concentration and sperm progressive motility (z=-0.183; p<0.05) (Spearman test) (Figure 1) (Figure 2) (Figure 3).

Figure 1 Correlation between seminal fructose concentration (g/L) and sperm concentration (billion/ml).

Figure 3 Correlation between seminal fructose concentration (g/L) and sperm progressive motility (%).
All 25cases azoospermia without seminal fructose have been examined and precede percutaneous epididymal sperm aspiration (PESA) by anthologist to find the infertility reason. The result shows that the reason in all the cases is Congenital Bilateral Absence of the Vas Deferens (CBAVD). Fructose concentration in 25cases with obstructive azoospermia is lower than normal or zero. By other side, 35cases with non-obtructive azoopermia, fructose concentration usually higher or equal than normal. In addition, there were 3patients with Klinefelter syndrome in group with non-obtructive azoospermia with elevated seminal fructose concentration.
Zinc concentration and seminal parameters
The Table 2 shows the following:

Figure 4 Correlation between seminal zinc concentration (mmol/L) and sperm progressive motility (%) (r=0, 596; p=0, 00001).
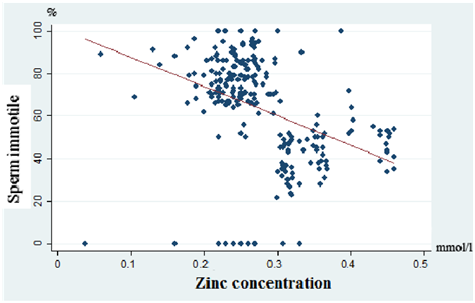
Figure 5 Correlation between seminal zinc concentration (g/L) and sperm immotile (%) (r=-0, 527; p=0, 0001).
The low zinc concentration group had an immotile percentage of 73.00±21.42% was higher than the normal zinc concentration group (44.07±15.43%) (z=10.433). This difference was statistically significant with p <0.001. Seminal zinc concentration showed a significant positive correlation (r=0.596) with sperm progressive motility (p<0.01) Negative correlations were observed with sperm immotile (r=-0.527) with statistical significance (p<0.01).
Group motility |
Low zinc concentration (mmol/L) (n = 84) |
Normal zinc concentration (mmol/L) (n = 96) |
z |
P |
Progressive motility (%) |
16.87±10,67 |
49.93±15,35 |
-11,481 |
0.00001 |
Non- progressive motility (%) |
3.64±2,07 |
4.07±4,63 |
-1,301 |
0.193 |
Immotile (%) |
73.00±21,42 |
44.07±15,43 |
10,433 |
0.0001 |
Table 2 Seminal zinc concentration and motility of the sperm
Correlation between seminal fructose and zinc with sperm DNA fragmentation
Sperm DNA fragmentation index in group with normozospermia correlated positively with seminal fructose (r=0.47, P<0.05) and negatively with seminal zinc (r=-0.56, P<0.05) concentrations. In group with oligozospermia found negative correlation (r=-0.24, P<0.05) between seminal fructose with sperm DNA fragmentation (Table 3).
Group |
r |
p |
Fructose concentration (g/L) and DFI (%) |
||
Oligozospermia (n=60) |
-0.24 |
<0.05 |
Normozospermia(n=60) |
0.47 |
<0.05 |
Zinc concentration (mmol/L) and DFI (%) |
||
Oligozospermia (n=60) |
-0.08 |
>0.05 |
Normozospermia (n=60) |
-0.56 |
<0.05 |
Table 3 Relationship between seminal fructose and zinc with sperm DNA fragmentation
Fructose is a main carbohydrate source in seminal plasma and necessary for sperm motion.20,21 The measurement of seminal fructose has been used in most laboratories. Therefore, the World Health Organization manual recommends measurement of seminal fructose as a marker of seminal vesicular function.22 Methods for determination of seminal fructose mainly includes gas chromatography, indole coloration, and resorcinol coloration. In particular, the resorcinol method has been used widely in clinical anthology laboratories for its simplicity of operation, high specificity, and no need for special instrument. Fructose in semen is the source of energy for all sperm activities. The higher of sperm concentration, and vitality and motility asked for more energy, so fructose is lower.9,23 Normal seminal fructose concentration confirms the role of testosterone and the function of vesicles and vas deferens are normal.24
In this study, negative correlations were observed between seminal fructose and sperm concentration (R=-0,156 và p>0,05), sperm vitality (R=-0,065 và p>0,05) and sperm progressive motility (R=-0,186 và p<0,05). This finding is in line with that of Gonzales13, Orakwe, et al.,25 and Mahmoud, et al.,26 Fructose in semen is the source of energy of every sperm activities. The higher of sperm concentration, vitality and motility asked for more energy, so fructose is lower.23,27 Lu (2007) reported seminal fructose concentration decreased, sperm concentration and mobility increased.23
Lewis Jones, et al.,6 found that fructose concentrations were inversely ratio to sperm motility with R=-,062 (p <0.05).6 However, Andrade Rocha FT (2001) confirmed that seminal fructose concentration was related to sperm concentration, survival, motility and morphology, but the results were not statistically significant.28 In the study of Amidu N (2012), seminal fructose concentration was negatively correlated with sperm motility (R=-0.04) but not statistically significant.29 Fructose concentrations were inversely ratio to sperm concentration (R=-0.21) with correlation was significant at 0.05 level.30 Fructose is the major glycolysable substrate of seminal plasma and is widely accepted as a marker of seminal vesicle function.29‒31 Inflammation may lead to atrophy of the seminal vesicles and low seminal fructose concentration. When ejaculatory ducts are blocked, fructose concentration in seminal plasma usually decreases and may become undetectable.29,32 Additionally, seminal plasma fructose concentration determination is useful for auxiliary diagnosis of obstructive and no obstructive azoospermia. Seminal fructose concentration in non-obstructive azoospermia is usually higher than or equal to that in males of normal fertility.33 However, fructose concentration in seminal plasma of patients with obstructive azoospermia is usually absent or significantly lower than that in men of normal fertility.29,31 Absence of seminal fructose has also been found in patients with congenital vas deferens-seminal vesicle developmental defect.18,34 Therefore, our results are consistent with most of the results of studies in the world.
Normal seminal fructose concentration confirms the role of testosterone and the function of vesicles and vas deferens are normal.31 The absence of both sperm and fructose correlates with the obstruction in CBAVD or retrograde ejaculation.1,13 Especially, the correlation between azoospermia and fructose in CBAVD had been proved by many authors.9 In this study, all 25cases azoospermia without seminal fructose have been examined and precede percutaneous epididymal sperm aspiration (PESA) by anthologist to find the infertility reason. The result shows that the reason in all the cases is CBAVD. Fructose concentration in obstructive azoospermia cases is lower than normal or absent.11 By other side, In human with non-obtructive azoopermia, fructose concentration usually higher or equal than normal.11 Inflammation the reproductive glands causes temporary obstruction, so that sperm count and seminal fructose concentration may decrease, but rarely absence both of them.11,35,36
One of the biochemical processes related to the genital fluid mixing is the regulation of the free seminal zinc fraction, which can interact with spermatozoa, such as, zinc and bioavailability. Zinc is first secreted in prostatic fluid in 2 forms available for sperm cells (free zinc and zinc-citrate complex). During ejaculation, however, a partial redistribution of the ion from citrate to very high affinity vesicular ligands reduces the unbound zinc fraction.37‒39 The measurement of zinc in human seminal plasma is important in the evaluation of male infertility. In present study the seminal plasma zinc levels were found to be immotile percentage in zinc concentration group was higher (73.00±21.42%) than the normal zinc concentration group (44.07±15.43%) (z=10.433).
A positive correlation between seminal plasma zinc levels and sperm concentration, motility was also observed in our study. This was in accordance with the previous studies of Fuse (1999), Chia (2000), Basil (2008), N. Amidu (2012).40‒42 Eliasson and Lindholme et al., in contrast could not find any correlation between zinc concentration and sperm density, motility or morphology.43 Some others authors not find the same results, but also agreed that zinc affects sperm motility. Omu (1998), Mahmoud Hussein Hadwan (2013), found that sperm motility increased after those subjects were given zinc supplements.44,45 Omar F. Abdul-Rasheed (2009), could not find correlation between zinc concentrations in semen and sperm mobility.46
Fuse et al. (1999) found positive correlation of zinc concentration with sperm concentration (r=0.33, p<0.05) and with sperm motility (r=0.22, p<0.05), while there was no correlation with sperm morphology and high zinc concentration is apparently related to defective motility in asthenozoospermic patients, even though adequate seminal plasma content of the element is required for normal sperm function.41 Mankad et al., found significant positive correlation was observed between zinc levels and sperm count (r=0.29, p<0.05) and zinc and alpha-glucosidase activity (r=0.31, p<0.05) in seminal plasma.47
Thus it seems that zinc is important for semen quality. The low zinc levels in the infertile men in our study might be attributed to disorders in the prostate excretory function or possibly due to asymptomatic prostate infection. Omu (1998), Mahmoud Hussein Hadwan (2013) and others found that sperm motility increased after treatment with zinc supplementation.48‒53 However, Omar F. Abdul-Rasheed (2009) found not correlation between zinc concentrations in semen and sperm motility.54
We also observed that mean % DFI is higher in the normozoospermic compared with the oligozoospermic men. Seminal fructose correlates positively with spermatozoa DNA fragmentation,55 and has been found to be both significantly up- and down-regulated in different aetiologies of male infertility.56,57 In this study, found that seminal fructose level (r=0.47, P<0.05) correlated positively with sperm DNA fragmentation index in group with normozospermia. In group with oligozospermia found negative correlation (r=-0.24, P<0.05) between seminal fructose with sperm DNA fragmentation. In addition, there were 3patients with Klinefelter syndrome in group with non-obtructive azoospermia with elevated seminal fructose concentration. Thus, the change in seminal fructose concentrations may reflect by high sperm DNA damage. Increased fructose levels in the group with azoospermia may be associated with Klinefelter's syndrome.
Seminal fructose concentration of normozopermia group is significant lower than oligozoospermia group. Fructose seminal concentration has a negative correlation with sperm concentration, and vitality and motility. Fructose is the major glycolysable substrate of seminal plasma and is widely accepted as a marker of seminal vesicle function. The progressive motility in the low zinc concentration group is significant lower than that of the normal zinc concentration group. The immotile in the low zinc concentration group is significant higher than that of the normal zinc concentration group. Zinc concentration has a positive correlation with sperm progressive motility and a negative correlation with immotile both are statistically significant. This is the first study to demonstrate that the increase of seminal fructose and decrease of zinc concentrations may reflected by high sperm DNA damage. This is the first report of increased in fructose concentration in seminal plasma from three azoospermic men’s with Klinefelter's syndrome.
The authors would like to take this opportunity to extend my sincere thanks to Ministry of Health for providing financial support for the study. We also are grateful for the technical support of the Hanoi Medical University Hospital for the assay of the seminal fructose and zinc concentration.
Author declares that there is no conflict of interest.

©2018 Trang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.