MOJ
eISSN: 2574-819X


Review Article Volume 2 Issue 5
Department of Chemistry, The College of New Jersey, USA
Correspondence: David A Hunt, Professor of Chemistry, Department of Chemistry, The College of New Jersey, 2000 Pennington Road, Ewing, NJ 08628, USA, Tel (609) 7713 174
Received: September 17, 2018 | Published: October 17, 2018
Citation: Hunt DA. Reaction of o-bromoaryl- and o-bromoaryll phthalimides with n-butyllithium at low temperatures. MOJ Biorg Org Chem. 2018;2(5):226-228. DOI: 10.15406/mojboc.2018.02.00086
In a study designed to determine chemoselectivity of nucleophilic addition versus bromine-lithium exchange, addition of n-butyllithium to o-bromoaryl- and o-bromo- arylalkyl phthalimides at low temperature results in clean addition to the imide carbonyl in lieu of bromine-lithium exchange thereby affording good to excellent yields of 3-n-butyl-3-hydroxyisoindolin-1-ones containing a bromoarene moiety. This methodology has potential for the preparation of a variety of highly functionalized nitrogen heterocycles.
Keywords: o-bromoaryl phthalimides, o-bromoarylalkyl phthalimides, bromine-lithium exchange studies, 2-bromoaryl-3-n-butylhydroxyisoindolin-1-ones, 2-bromoarylalkyl-3-n-butylhydroxyisoindolin-1-ones
The work of Parham, Jones and Sayed,1 which demonstrates that bromine-lithium exchange, occurs in preference to carbonyl addition in aromatic amide derivatives of o-bromo-b-phenylpropionic acid, led to the consideration of using o-bromoaryl phthalimides 1a-c as precursors for the preparation of multi-ring nitrogen heterocycles and for elaborations of aromatic systems requiring an amine-protecting group (Figure 1). While the chemistry of the addition of Grignard and organolithium reagents to phthalimides is well known,2 to the best of our knowledge, attempts to carry out the addition of the these reagents to brominated phthalimide derivatives such as 1a-c have not been studied.
Even at low temperatures (ca. -100°C), bromine-lithium exchange to provide intermediate 2 proved fruitless in each case, presumably due to the greater electro- philicity of the imide carbonyl system compared to the aryl bromide. The major product cleanly formed in each case was the addition product arising from attack of the n-butyl-lithium on the imide carbonyl group (Compound 3a-c, Figure 2).
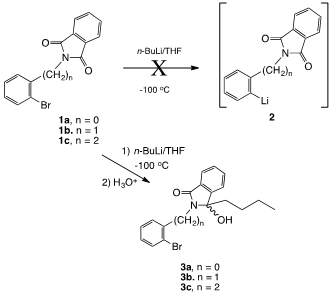
Figure 2 The major product cleanly formed in each case was the addition product arising from attack of the n-butyl-lithium on the imide carbonyl group.
While the reaction of Grignard reagents with phthalimides is well known and has proven to be a valuable method for the preparation of alkylidenephthalimidines,3 heating is typically necessary for the reaction. In the case of the low temperature exchange attempts of these systems, aliquotting experiments revealed that substantial butylation had occurred after 30minutes at -100°C. Compound 3a was determined to be a mixture of rotamers based on 13C NMR analysis, presumably due to steric inhibition of free rotation about the phenyl C-N bond. The homologs 3b and 3c did not exhibit this behavior based of 13C NMR analysis. The 1H NMR spectrum of 3b revealed the presence of an AB pattern for the benzylic protons. Presumably, this is not due to rotational barriers since this was not observed in the 13C NMR spectrum.
General
Melting points were determined on a Mel-Temp heating block in open capillary tubes and are uncorrected. 1H NMR spectra were obtained on a Varian Gemini 300MHz NMR with tetramethylsilane as an internal reference. 13C NMR spectra (75MHz) were obtained utilizing CDCl3 lock. IR data were recorded on a Perkin-Elmer 2000 FT-IR spectrometer. Elemental analyses were performed by MHW Laboratories, Phoenix, AZ. All starting material, reagents, and solvents were reagent grade and were used without additional purification. Tetrahydrofuran was dried over lithium aluminum hydride.
Preparation of N-(o-bromophenyl) phthalimide (1a)4
To a 500mL Erlenmeyer flask equipped with a magnetic stirrer were added o-bromo-aniline (20.40g; 0.119mol), phthalic acid (19.69g; 0.119mol), and acetic acid (150mL) and the resulting mixture was heated with stirring to 100°C (oil bath) for 14h. The mixture was then allowed to cool and was poured into water (400mL). The resulting crystalline precipitate was collected by vacuum filtration and was air-dried to constant weight (32.83g, 91%). The compound was purified by recrystallization from ethanol-chloroform (1:1) to provide purified 1a as white needles (31.24 g, 87%), mp 126-27.5°C; IR (KBr): 1675cm-1; 1H NMR (300MHz, CDCl3): d7.22-8.12 (m, ArH). Anal. Calcd for C14H8BrNO2: C, 55.63; H, 2.65; Br, 26.49; N, 4.64. Found: C, 55.65; H, 2.79; Br, 26.57; N, 4.48.
Preparation of N-(o-bromobenzyl) phthalimide (1b)5
To a solution of o-bromobenzyl bromide (32.45g; 0.13mol) in DMF (75mL) in a 500mL Erlenmeyer flask equipped with a magnetic stirrer was added potassium phthalimide (25.90g; 0.14mol). An exothermic reaction ensued (70°C within 8min), and stirring was continued until the mixture cooled to room temperature (4h). The mixture was then diluted with chloroform (200mL) and the resulting mixture was poured into water (300mL). The aqueous phase was separated and was extracted with chloroform (2x50mL). The combined organics were then washed sequentially with aqueous NaOH (0.2N, 300mL) and water (300mL). The organics were dried, filtered, and concentrated in vacuo to afford 1b (33.47g, 82%) as a brown semisolid, which was triturated with diethyl ether to provide fine white needles (32.00 g, 78%), mp 162-64°C; IR (KBr): 1609 cm-1; 1H NMR (300 MHz, CDCl3): d5.09 (s, 2, benzylic CH2), 7.12-7.43 (m, 3 ArH), 7.50-7.67 (m, 1 ArH), 7.80-8.12 (m, 4, ArH). Anal. Calcd for C15H10BrNO2: C, 56.96; H, 3.16; Br, 25.32; N, 4.43. Found: C, 56.84; H, 3.12; Br, 25.41; N, 4.27.
Preparation of N-(o-bromo-b-phenylethyl) phthalimide (1c)6
o-Bromo-b-phenylethyl bromide (90.65g; 0.343mol) was place in a 1L Erlenmeyer flask equipped with a magnetic stirrer and oil bath and was dissolved in DMF (280mL). To the stirred solution was added potassium phthalimide (66.64g; 0.360mol) and the resulting mixture was heated to 90°C with stirring for 15h. The mixture was allowed to cool and was diluted with chloroform (400mL). The mixture was transferred to a seperatory funnel containing water (300mL). After vigorous mixing, the aqueous phase was removed and extracted with chloroform (2x50mL). The combined chloroform layers were washed sequentially with aqueous NaOH (0.2N, 300mL) and water (300mL). The organics were dried, filtered, and concentrated in vacuo to afford 1c as a pale yellow solid (94.27 g, 83%) recrystallized from 7:3 ligroin-methanol to provide fine white needles, mp 96.5-98°C; IR (KBr): 1690cm-1; 1H NMR (300MHz, CDCl3): d3.16 (t, 2, J=8Hz, CH2), 4.02 (t, 2, J=8Hz, benzylic CH2), 7.00-7.96 (m, 8, ArH). Anal. Calcd for C16H12BrNO2: C, 58.18; H, 3.64; Br, 24.24; N, 4.24. Found: C, 58.06; H, 3.60; Br, 24.43; N, 4.04.
General procedure for the reaction of n-butyllithium with N-(o-bromoaryl) phthalimides. Preparation of Compounds 3a-c
The bromoaryl phthalimide (1a-c) was dissolved in anhydrous THF (125mL) and hexane6 (30mL), and the resulting solution was placed into a three-neck 250mL round bottom flask equipped with an overhead stirrer, addition funnel, low temperature thermometer and N2 inlet, and low temperature (LN2/diethyl ether) bath. The mixture was cooled to -100°C, and n-butyllithium (1.0molar equivalent) was added at such a rate so as to maintain the temperature below -95°C. After the addition was complete, the mixture was stirred at -100°C for 1h. The mixture was then allowed to warm to room temperature (2h) and was poured into water (200mL). The organic phase was separated from the aqueous phase and the aqueous phase was extracted with diethyl ether (3x125mL). The combined organics were dried (MgSO4), filtered and concentrated in vacuo. The crude products were purified by recrystallization.
2-(2-Bromophenyl)-3-butyl-3-hydroxyisoindolin-1-one (3a) (mixture of rotamers) was isolated as white needles (3.45g, 64%) after recrystallization from benzene-petroleum ether (1:4) by reaction of N-o-(bromophenyl) phthalimide (1a, 4.53g; 0.015mol) with n-butyllithium (6.52mL; 0.015mol/2.3M in hexane), mp 146-46.5°C; IR (KBr): 3360, 3050, 2925, 1665, 1610, 1470, 1380, 1110, 1020, 860, 830, 760cm-1; 1H NMR (300MHz, CDCl3): d0.82 (t, 3, J=7Hz, CH3), 1.02-1.46 (m, 4, CH2CH2), 1.80-2.22 (m, 2, CH2), 2.96 (bd s, 1, OH), 7.08-7.82 (m, 8 ArH); 13C NMR (75MHz, CDCl3): d18.40, 29.80, 30.04, 34.60, 48.00, 48.50, 93.78, 94.47, 122.46, 123.10, 124.36, 124.79, 126.78, 128.68, 128.76, 129.29, 130.16, 130.33, 130.69, 131.24, 132.25, 133.39, 134.01, 134.73, 134.95, 135.31, 135.83, 148.21, 148.74, 166.92, 167.92. Anal. Calcd for C18H18BrNO2: C, 60.00; H, 5.00; Br, 22.22; N, 3.89. Found: C, 59.92; H, 5.09; Br, 21.98; N, 3.60.
2-(2-Bromobenzyl)-3-butyl-3-hydroxyisoindolin-1-one (3b) was isolated as white needles (3.20 g, 86%) after recrystallization from ligroin-toluene (3:1) by reaction of N-o-(bromobenzyl) phthalimide (1b, 3.16g; 0.01=mol) with n-butyllithium (4.35mL; 0.01mol/2.3M in hexane), mp 161.5-62°C; IR (KBr): 3180, 2925, 1640, 1610, 1390, 1310, 1260, 1110, 1070, 1020, 950, 890, 870, 730, 700cm-1; 1H NMR (300MHz, CDCl3): d0.58-1.08 (m, 7, CH3CH2CH2), 1.87-2.16 (m, 2, CH2), 3.97 (s, 1, OH), 4.60 (AB pattern, 2, J=15Hz, benzylic CH2), 7.03-7.97 (m, 8, ArH); 13C NMR (75MHz, CDCl3): d13.58, 22.22, 25.53, 35.93, 41.39, 91.81, 112.39, 121.89, 122.93, 123.51, 127.48, 128.71, 129.56, 130.08, 130.66, 132.55, 137.09, 146.90, 168.02. Anal. Calcd for C19H20BrNO2: C, 60.96; H, 5.35; Br, 21.39; N, 3.74. Found: C, 60.99; H, 5.40; Br, 21.44; N, 3.49.
2-(2-Bromophenylethyl)-3-butyl-3-hydroxyisoindolin-1-one (3c) was isolated as white needles (3.50 g, 90%) after recrystallization from toluene by reaction of N-o-(bromo-b-phenylethyl) phthalimide (1c, 3.30 g; 0.01mol) with n-butyllithium (4.35mL; 0.01mol/2.3M in hexane), mp 97.5-98.5°C; IR (KBr): 3225, 3050, 2950, 1640, 1600, 1310, 1280, 1060, 1010, 750cm-1; 1H NMR (300MHz, CDCl3): d0.77-1.32 (m, 7, CH3CH2CH2), 2.11 (t, 2, J=8Hz, CH2), 3.03-3.80 (m, 4, CH2, benzylic CH2), 3.76 (bd s, 1, OH), 6.92-7.57 (m, 8, ArH); 13C NMR (75MHz, CDCl3): d13.84, 22.42, 25.60, 35.09, 35.87, 38.66, 91.42, 121.69, 123.12, 124.55, 126.31, 127.61, 128.19, 129.36, 131.11, 132.22, 132.81, 138.65, 146.90, 167.76. Anal. Calcd for C20H22BrNO2: C, 61.86; H, 5.67; Br, 20.62; N, 3.61. Found: C, 62.04; H, 5.78; Br, 20.77; N, 3.43.
Hydroxphthalimidines 3a-c were obtained in good yields with no perceptible halogen-metal exchange by low temperature addition of n-butyllithium to N-(o-bromophenyl)- phthalimide and N-(o-bromophenylalkyl)phthalimides and were characterized by 1H and 13C NMR, IR, and elemental analysis. This methodology may provide an entry to a variety of heterocyclic systems that may utilize the latent functionalization that subsequent bromine-lithium exchange can provide.
None.
Author declares that there is no conflict of interest.

©2018 Hunt. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.