MOJ
eISSN: 2574-819X


Research Article Volume 1 Issue 4
1Department of Chemistry, Karunya University, India
1Analytical Science discipline, CSMCRI (CSIR), India
Correspondence: Paulraj Mosae Selvakumar, Department of Chemistry, Karunya University, Coimbatore, Tamilnadu, India-641114
Received: August 22, 2017 | Published: September 20, 2017
Citation: Selvan GT, Chitra V, Enoch IMV, et al. 1-(1-hydroxynaphthalen-2-Yl) ethanone: crystal structure, photo physical study and turn off molecular switch with cu (ii) ion. MOJ Biorg Org Chem. 2017;1(4):136-139. DOI: 10.15406/mojboc.2017.01.00025
A simple 1-naphthol derivative, 1-(1-hydroxynaphthalen-2-yl) ethanone (1-NAPH) was used for selective detection of Cu2+ ions and the X-ray single-crystal structure was reported. The structure is stabilized by intramolecular H-bonding and by intermolecular C-H···π and π-π stacking interactions. Study of the photo physical behavior of naphthol derivative towards Cu2+ ion was explored in methanol medium. When titrated with various metal ions, the fluorescence emission of 1-NAPH was quenched in the presence of Cu2+ ion. Hence, 1-NAPH acts as a molecular switch towards Cu2+ ions due to its fluorescence turn “OFF” behavior.
Keywords: naphthol, crystal structure, cu2+detection, fluorescence quenching, molecular switch
Detection of metal cations has received special attention since they play important role in the living system. To detect and control metal ions in aqueous solution is also very important. Among the various transition metal ions, copper (II) ion is a significant environmental pollutant depending on its concentration while it is an essential trace element in biological systems.1 Deficiency of copper leads to Menkes disease, but high levels can cause Alzheimer2 or Parkinson’s disease3 and kidney damage. Cu2+ ion can be detected using various instrumental techniques. However, most of the methods are time-consuming and requires expensive instrumentation techniques.4-9 Therefore, development of a selective, sensitive and easy method10-14 to detect Cu2+ ions becomes essential. Cu2+ ion is a fluorescent quencher due to its paramagnetic nature and so a fluorescent receptor responds towards Cu2+ ion can act as molecular switches.15-17
Naphthaldehyde, hydroxy-naphthaldehyde and their derivatives are an important class of intermediates which condenses with primary amines to afford Schiff bases that are one of most versatile mixed-donor ligands in the field of coordination chemistry. In this paper, we have reported1-(1-hydroxynaphthalen-2-yl)ethanone, 1-NAPH and its photophysical studies for the detection of Cu2+ ions in aqueous medium. 1-NAPH acts as on-off-on molecular switch towards Cu2+ ions due to its fluorescence Turn “OFF” behavior. The crystal structure of the receptor and its molecular switch behavior with Cu2+ ion was also explored.
Reagents
All reagents and chemicals, unless stated otherwise, were purchased from commercial suppliers and used without further purification. 1-Hydroxy-2-acetonaphthone was purchased from Aldrich. All inorganic salts used were of analytical grade. Thrice distilled water was used throughout the experiments. Ortho phosphoric acid buffer solutions were prepared and used for pH studies.
Instrumentation
UV-Visible and fluorescence spectral studies were recorded using a double-beam Jasco V630 spectrophotometer and a Jasco FP-8300 spectrofluorometer respectively using cuvettes of 1.0 cm path length. The spectrofluorometer used a 120 W Xenon lamp as the excitation source and the excitation and emission bandwidths were fixed at 5.0 nm. A crystal of suitable size was selected and mounted on the tip of a glass fiber and cemented using epoxy resin Intensity data for crystal were collected using Mo-Ka (l = 0.71073Å) radiation on a Bruker SMART APEX diffractometers equipped with CCD area detector at 100K. An empirical absorption correction was applied to the collected reflections with SADABS.18 The structures were solved by direct methods using SHELXTL19 and were refined on F2 by the full-matrix least-squares method using the SHELXL-9720 package. Graphics are generated using PLATON21 and MERCURY 1.3.22 All non-hydrogen atoms were refined anisotropically till convergence is reached. Hydrogen atoms in the compound were fixed at idealized positions stereochemically.
Preparation of single crystal
1-Hydroxy-2-acetonaphthone was crystallized in methanol solution. Pale-yellow needle-like single crystals suitable for X-ray diffraction studies were obtained after several weeks by slow evaporation from methanol-ethyl ether (1:3) mixed solution.
UV-Visible and fluorescence Spectroscopic studies
Standard stock solution of 1-NAPH (1 x 10-3 M) was prepared in methanol. Various metal ion solution (1 x 10-3 M) viz., Eu3+, Pb2+, Cu2+, Ni2+, Cd2+, Na+, Ca2+, Mn2+, Ba2+, Mg2+, NH4+, Li+, Al3+, Hg2+, K+, Fe2+ and Zn2+ were prepared by dissolving the exactly weighed amount of the metal salts in standard measuring flask. Working solutions were prepared by diluting the stock solutions. The spectrum was recorded by adding various metal ion solution to 1-NAPH. Standard measuring flasks containing the test solutions were thoroughly mixed for few minutes at room temperature before recording UV-Vis and fluorescent spectra.
Preparation of various pH solutions
Solutions of various pH 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 were prepared by mixing appropriate amounts of orthophosphoric acid and NaOH. Solutions of 1-NAPH and Cu2+ were diluted in various pH solutions pH 2.0-12.0 and its UV-Visible and Fluorescence spectra were recorded. All pH values were measured with Elico 2F 120 (India) pH meter.
Single crystal structure
Suitable single crystal for compound 1-NAPH was obtained upon slow evaporation at room temperature in a week. Compound 1-NAPH crystallizes in Space group P 21/n (a=7.4808(12), b= 6.9867(11), c= 17.450(3)). The ORTEP view of the compound1-NAPH is depicted in Figure 1. X-Ray crystallographic analysis revealed the role of intermolecular hydrogen bonding in the compound and the structure is shown in Figure 2. An intramolecular O2-H2···O1hydrogen bond (distance O...H=1.806Å, O-H=0.820Å, bond angle=146.64°) between the hydroxyl group and the O atom of carbonyl group forms a six-membered ring in each molecule, which is nearly coplanar with the naphthone ring and the distances from C1 atom of acetyl group to mean plane of the three six-membered ring is 0.112(3) Å. There are weak intermolecular π-π stacking interactions between neighbouring aromatic rings (C3-C8) with centroid-to-centroid distances of 3.844(2) Å (Figure 2). Moreover, the packing structure is stabilized by intermolecular C-H···π stacking interactions.
UV-Visible and fluorescence studies
1-NAPH was screened with a series of metal cations such as Li+, Na+, K+, Eu3+, Fe2+, Hg+, NH4+, Cu2+, Cd2+, Ni2+, Ba2+, Mn2+, Al3+, Mg2+, Pb2+, Ca2+ and Zn2+ respectively. The UV-visible spectra of 1-NAPH in the presence and absence of metal ions showed two broad bands at 255 and 367 nm with shoulders around 290-300 nm was observed (Figure 3A). Upon addition of Cu2+ ion, the shoulders around 290-300 got disappeared and a hypochromic shift was observed at 255 nm in the blue shift region. This clearly demonstrates that 1-NAPH was strongly bound to Cu2+ ions. Figure 3B shows the fluorescence spectra of 1-NAPH with various metal ions in methanol-water medium.1-NAPH showed a strong fluorescence at 496 nm in the presence and absence of various metal ions, when excited at 367 nm. In contrast, the fluorescence of 1-NAPH was almost quenched after addition of 20 µmol/L Cu2+ ions.

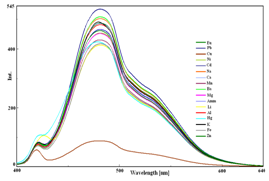
Figure 3 Changes in the (A) absorption spectra and (B) fluorescence spectra of 1-NAPH in the presence of various metal ions.
The highly sensitive nature of 1-NAPH towards Cu2+ ion was shown in Figure 4A & 4B. A decrease in absorbance was observed with increasing concentration of Cu2+ ions. In fluorescence emission spectrum, 1-NAPH exhibited a strong fluorescence at 496 nm. Upon gradual addition of increasing amounts of aqueous Cu2+ ion solution, the fluorescence emission at 496 was quenched. This showed the strong interaction between 1-NAPH and Cu2+ ions.
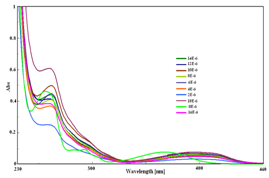

Figure 4 Changes in (A) Absorption and (B) emission spectra of 1-NAPH upon gradual addition of Cu2+ ions.
Solvent effect
Absorbance and fluorescence emission spectra (Figure 5A & 5B) of 1-NAPH were recorded in different solvents viz., ethanol, dimethyl sulfoxide, dimethyl formamide, chloroform and acetonitrile of total concentration 10 µM. UV spectrum of 1-NAPH with various solvents shows increase in polarity of solvents lead to hyperchromic shift of the absorption bands for the corresponding solvents. Emission spectra showed that maximum fluorescence intensity was observed for DMF.
pH effect
The effect of pH of the solution, in which the sensor is applied, is a critical factor that must be considered definitely. The study of effect of pH of 1-NAPH towards Cu2+ ion was investigated from pH range 2.0-12.0. As shown in the Figure 6, UV-Visible spectra showed gradual decrease in absorbance with increasing pH. Under neutral pH, the absorbance of 1-NAPH was quenched maximally. Evidently, the binding between hydroxyl group and Cu2+ caused this pronounced decrease, which also demonstrated the recognition ability of hydroxyl group toward Cu2+. In acidic pH range, the hydroxyl group of 1-NAPH was protonated and so decreases the binding of 1-NAPH with Cu2+ ions. In basic range from pH 10-12, a red shift was observed due to the strong binding between 1-NAPH and Cu2+ ion.
Proposed binding mode
Based on the above studies, we proposed a binding mode between 1-NAPH and Cu2+ ion (Scheme 1). The X-ray crystal study of 1-NAPH confirmed the presence of intramolecular hydrogen bonding between the hydroxyl group and the O atom of carbonyl group forms a six-membered ring in each molecule. The interaction of Cu2+ with 1-NAPH includes the annihilation of the hydrogen bonding and so Cu2+ ion is bound to the oxygen atom of the hydroxyl and the oxygen atom of the carbonyl group.
Single crystal structure of 1-NAPH was studied by X-ray diffraction method which revealed the existence of intramolecular hydrogen bonding. The structure is stabilized by intramolecular H-bonding and by intermolecular C-H···π and π-π stacking interactions.1-NAPH acts as a selective fluorescent receptor for Cu2+ ions in aqueous medium. The fluorescence emission of 1-NAPH was quenched in the presence of Cu2+ ions upon excitation at 367 nm. When titrated with various metal ions, the fluorescence emission of 1-NAPH was quenched in the presence of Cu2+ ion. We conclude that 1-NAPH acts as on-off-on molecular switch towards Cu2+ ions due to its fluorescence Turn “OFF” behavior.
P.M.S.K. is thankful to the Science and Engineering Research Board (SERB), Department of Science and Technology, India, for the financial support (Project number SR/FT/CS-068/2012).
The author declares no conflict of interest.

©2017 Selvan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.