MOJ
eISSN: 2573-2951


Research Article Volume 5 Issue 3
1Department of Chemistry, Government College University, Pakistan
2College of Natural Sciences, Department of Biological Science, Kongju National University, South Korea
3Faculty of Pharmacy & Atta-ur-Rahman Institute for Natural Products Discovery (AuRIns), Universiti Teknologi MARA, Malaysia
4Department of Biochemistry, University of Agriculture, Pakistan
Correspondence: Muhammad Athar Abbasi, Department of Chemistry, Government College University, Lahore-54000, Pakistan, Ext 266, Tel (+92)-42-111000010
Received: December 04, 2017 | Published: May 9, 2018
Citation: Abbasi MA, Mumtaz A, Aziz-ur-Rehman, et al. Hemolytic profile of novel tri-heterocyclic benzamides. MOJ Bioequiv Availab. 2018;5(3):138-142. DOI: 10.15406/mojbb.2018.05.00094
Heterocyclic compounds containing five-membered or six-membered heterocyclic units have a diversity of valuable biological effects. In the present work, some novel tri-heterocyclic benzamides, 8a-g, were synthesized in multi-steps. The benzamide containing electrophile, {4-[4-(chloromethyl)benzoyl]-1-piperazinyl}(2-furyl) methanone (3), was synthesized by the reaction of 4-(chloromethyl)benzoyl chloride (2) and 2-furoyl-(1-piperazinyl) methanone (1) in a basic aqueous medium. In parallel series of steps, substituted-benzoic acids (4a-g) were refluxed with ethanol and conc. sulfuric acid to form respective ethyl substituted-benzoates (5a-g). These esters were further refluxed with N2H4.H2O in methanol solution to acquire substituted-benzohydrazides (6a-g). These hydrazides were cyclized into heterocyclic core by refluxing with CS2 in the presence of KOH and ethanol solvent, whereby yielding various 5-(substituted-phenyl)-1,3,4-oxadiazol-2-thiols (7a-g). In the final step, the electrophile 3, was refluxed with synthesized 1,3,4-oxadiazoles, 7a-g, in acetonitrile and potassium carbonate to acquire the targeted novel tri-heterocyclic benzamides, 8a-g. The structural characterization of these newly synthesized molecules was done by IR, 1H-NMR, 13C-NMR, and EI-MS spectral data. All these compounds were evaluated for their hemolytic activity to ascertain their cytotoxicity profile.
Keywords: novel tri-heterocycles, 1H-NMR, 13C-NMR, EI-MS, hemolytic activity, heterocyclic compounds
OLED, organic light-emitting diodes; KOH , potassium hydroxide; NMR, nuclear magnetic resonance; TLC, thin layer chromatography; EDTA, ethylene diamine tetra acetic acid; PBS, Phosphate-buffered saline; HEC, higher education commission; EI-MS, electron ionization mass spectrometry
Oxadiazoles are the heterocyclic compounds containing one oxygen and two nitrogen atoms in a five membered ring,1 possessing a diversity of useful biological effects.2 Oxadiazole is considered to be resultant from furan by replacement of two methane (–CH=) groups by two pyridine type nitrogen atoms (–N=) at position 3 and 4.3 Oxadiazole is a very weak base due to the inductive effect of the extra heteroatom.4 The replacement of (–CH=) groups in furan by two pyridine type nitrogen (–N=) reduces aromaticity of the resulting oxadiazole ring to such an extent that the oxadiazole ring exhibits the character of conjugated diene.5 Due to relatively low electron density on the carbon atom, the oxadiazole ring is extremely resistant towards electrophillic substitutions at carbon atom; however the attack of electrophile occurs at nitrogen, if oxadiazole ring is substituted with electron releasing groups. Nucleo-philic attack is quite difficult in oxadiazole ring; however, halogen substituted oxadiazoles can undergo nucleophilic substitution with replacement of halogen atom by nucleophiles.6 These derivative compounds have been found to exhibit diverse biological activities such as analgesic,7 anti-inflammatory,8 antimicrobial,9 anti-HIV,10 antimalarial,11 antifungicidal,12 and other biological properties. Some 1,3,4-oxadiazole derivatives have also been applied in the fields of photosensitizers,13 liquid crystals,14 and organic light-emitting diodes (OLED).15 Consequently, the synthesis of compounds containing this heterocyclic core has attracted considerable attention, and a wide variety of methods has been used for their assembly. The most common synthetic protocol toward the preparation of these compounds involves the dehydrative cyclization of diacylhydrazides using usually strong acidic reagents such as thionyl chloride,16 phosphorus pentoxide,17 phosphorus oxychloride,18 and sulfuric acid.19
Literature survey showed that slight modifications in the structure of 1,3,4-oxadiazole can result in quantitative as well as qualitative variations in the biological activity.20 So, in the present study we have synthesized various tri-heterocyclic benzamides through a multi-step process to incorporate multi-functionalities in their skeleton. Then, cytotoxicity of these molecules was profiled through hemolytic study on the membrane of red blood cells.
Chemistry
All the chemicals, along with analytical grade solvents, were purchased from Sigma Aldrich, Alfa Aesar (Germany), or Merck through local suppliers. Pre-coated silica gel Al-plates were used for TLC with ethyl acetate and n-hexane as solvent system. Spots were detected by UV254. Gallonkamp apparatus was used to detect melting points in capillary tubes. IR spectra (νmax, cm–1) were recorded by KBr pellet method in the Jasco-320-A spectrophotometer. 1H-NMR spectra (δ, ppm) were recorded at 600 MHz (13C-NMR spectra, at 150 MHz) in CDCl3 using the Bruker Advance III 600 As- cend spectrometer using BBO probe. EI-MS spectra were measured on a JEOL JMS-600H instrument with data processing system.
Procedure for the preparation of {4-[4-(chloromethyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (3)
2-Furyl(1-piperazinyl)methanone (12.8mmol; 1) was taken in an iodine flask (250mL) containing 15mL of distilled water and 10% Na2CO3 (sodium carbonate) solution to adjust pH at 9-10. Then equimolar 4-(chloromethyl)benzoyl chloride (2) was added dropwise to the reaction medium in 2-5 min. After complete addition, the iodine flask was vigorously shaken (manually) and then set to stir at room temperature for 4 h till the formation of solid precipitates. The progress of reaction was monitored by thin layer chromatography (TLC) till single spot. The obtained precipitates were filtered, washed with distilled water and dried to yield the titled electrophiles.20,21
Procedure for the preparation of ethyl substituted-benzoates (5a-g)
Substituted-benzoic acids (50mmol; (4a-g, one in each reaction) were taken into a 250mL round bottom flasks with a reflux condenser, then absolute ethanol (40mL) and conc. sulphuric acid (1/2mL) were added into the flask and the reaction mixture was refluxed for 3-4h. After maximal completion by thin layer chromatography (TLC), excess water was added and pH was adjusted to 8-10 by aqueous solution of sodium carbonate (Na2CO3; 10%). The title compounds were extracted by chloroform.
Procedure for the preparation of substituted-benzohydrazides (6a-g)
Esters, 5a-g, (40mmol) and N2H4.H2O (hydrazine monohydrate; 40mmol) were taken in a 100mL round bottom flask with a reflux condenser and were refluxed for 4-6h with 25mL methanol. After final thin layer chromatography (TLC), excess water was added to acquire precipitates which were separated by filtration.
Procedure for the preparation of 5-(substituted-phenyl)-1,3,4-oxadiazol-2-thiols (7a-g)
Acid hydrazides, 6a-g, (30mmol) were refluxed for 1/2 h with solid KOH (potassium hydroxide; 30mmol) in 45mL ethanol in a 100mL round bottom flask. Then CS2 (carbon disulfide; 60mmol) was added and further refluxed for 3-6 h. After final thin layer chromatography (TLC), excess water was added followed by conc. HCl to adjust pH of 2. The mixture was left for 3h for precipitation. Precipitates were collected through filtration and washed with water.
General procedure for the preparation of {4-[4-({[5-(substituted-phenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (8a-g)
5-(Substituted-phenyl)-1,3,4-oxadiazol-2-thiols (24mmol; 7a-g, one in each reaction) were dissolved in acetonitrile (20-30mL) in 100mL round bottom flask. Then solid K2CO3 (potassium carbonate; 12mmol) was added. The mixture was refluxed for 1/2 h and then the equimolar (24mmol) desired electrophile, 3 were added. The mixture was further refluxed for 4-5 hours. Thin layer chromatography (TLC) was carried out to check the reaction completion. Distilled water was added to the reaction mixture to acquire the precipitates. Precipitates were filtered, washed and dried to get the titled compounds.8,17
{4-[4-({[5-(2-Chlorophenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (8a)
Light brown liquid; yield: 85%; Molecular formula: C25H21ClN4O4S; molecular mass: 508g/mol; IR (KBr, υmax cm–1): 3415 (N-H), 3063 (Ar C-H), 2872 (C-H), 1654 (C=O), 1576 (C=C), 1200 (C-O-C), 1112 (C-N-C), 644 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): δ 7.93 (d, J = 6.0 Hz, 2H, H-2'' & H-6''), 7.55 (d, J = 6.6 Hz, 2H, H-3'' & H-5'') 7.52 (br s, 1H, H-5), 7.42-7.39 (m, 4H, H-3'''', H-4'''', H-5'''' & H-6''''), 7.07 (d, J = 3.4 Hz, 1H, H-3), 6.50 (dd, J = 1.7, 3.5 Hz, 1H, H-4), 4.54 (s, 2H, H-8''), 3.82 (br s, 4H, H-3' & H-5'), 3.46 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.09 (C-7''), 164.33 (C-5'''), 164.15 (C-2'''), 159.2 (C-6), 147.65 (C-2), 143.96 (C-5), 137.96 (C-4''), 134.95 (C-1''''), 133.03 (C-1''), 132.45 (C-6''''), 131.3 (C-4''''), 130.92 (C-2''''), 129.48 (C-5''''), 127.66 (C-3'' & C-5''), 127.11 (C-3''''), 122.81 (C-2'' & C-6''), 117.26 (C-3), 111.54 (C-4), 52.00 (C-2', C-3', C-5' & C-6'), 36.27 (C-8''); EI-MS (m/z): 383 [M]+, 259 [C16H25N2O]+, 261 [C15H21N2O2]+, 204 [C13H18NO]+, 193 [C10H13N2O2]+, 150 [C9H12NO]+, 134 [C8H8NO]+, 107 [C7H9N]•+, 92 [C7H8]•+, 95 [C5H3O2]+.
{4-[4-({[5-(3-aminophenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (8b)
Light brown liquid; yield: 81%; Molecular formula: C25H23N5O4S; molecular mass: 489g/mol; IR (KBr, υmax cm–1): 3413 (N-H), 3071 (Ar C-H), 2886 (R C-H), 1658 (C=O), 1580 (Ar C=C), 1203 (C-O-C), 1106 (C-N-C), 653 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): δ 7.96 (d, J = 7.9 Hz, 1H, H-4''''), 7.59 (s, 1H, H-2''''), 7.53-7.49 (m, 2H, H-2'' & H-6''), 7.50 (d, J = 8.1 Hz, 1H, H-6''''), 7.49 (br s, 1H, H-5), 7.42 (t, J = 7.6 Hz, 1H, H-5''''), 7.30 (d, J = 7.8 Hz, 2H, H-3'' & H-5''), 7.07 (d, J = 2.0 Hz, 1H, H-3), 6.50 (dd, J = 1.6, 3.4 Hz, 1H, H-4), 4.64 (s, 2H, H-8''), 3.85 (br s, 4H, H-3' & H-5'), 3.52 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.09 (C-7''), 164.97 (C-2'''), 164.49 (C-5'''), 159.23 (C-6), 147.63 (C-2), 143.81 (C-5), 137.28 (C-4''), 133.56 (C-1''), 132.06 (C-3''''), 131.7 (C-1''''), 130.38 (C-2''''), 129.29 (C-4''''), 128.15 (C-5''''), 127.88 (C-3'' & C-5''), 126.92 (C-6''''), 122.8 (C-2'' & C-6''), 117.56 (C-3), 111.87 (C-4), 45.50 (C-2', C-3', C-5' & C-6'), 36.76 (C-8''); EI-MS (m/z): 489 [M]+, 421 [C21H19N5O3S]•+, 392 [C20H18N5O2S]+, 310 [C16H12N3O2S]+, 246 [C13H14N2O3]•+, 243 [C12H9N3OS]•+, 179 [C9H11N2O2]+, 161 [C8H7N3O]+, 151 [C8H9NO2]+, 95 [C5H3O2]+.
6{4-[4-({[5-(3-nitrophenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (8c)
Brick red liquid; yield: 82%; Molecular formula: C25H21N5O6S; molecular mass: 519g/mol; IR (KBr, υmax cm–1): 3414 (N-H), 3087 (Ar C-H), 2870 (R C-H), 1665 (C=O), 1579 (Ar C=C), 1190 (C-O-C), 1114 (C-N-C). 645 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): δ 7.53-7.49 (m, 2H, H-2'' & H-6''), 7.49 (br s, 1H, H-5), 7.44 (s, 1H, H-2''''), 7.41-7.39 (m, 3H, H-4'''', H-5'''' & H-6''''), 7.30 (d, J = 7.8 Hz, 2H, H-3'' & H-5'') 7.07 (d, J = 2.0 Hz, 1H, H-3), 6.50 (dd, J = 1.6, 3.4 Hz, 1H, H-4), 4.64 (s, 2H, H-8''), 3.85 (br s, 4H, H-3' & H-5'), 3.52 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.05 (C-7''), 164.34 (C-5'''), 164.17 (C-2'''), 159.23 (C-6), 149.53 (C-3''''), 147.6 (C-2), 143.91 (C-5), 137.99 (C-4''), 133.09 (C-1''), 130.78 (C-6''''), 129.23 (C-5''''), 128.48 (C-1''''), 127.66 (C-3'' & C-5''), 126.04 (C-4''''), 125.4 (C-2''''), 122.8 (C-2'' & C-6''), 117.26 (C-3), 111.55 (C-4), 48.05 (C-2', C-3', C-5' & C-6'), 36.23 (C-8''); EI-MS (m/z): 397 [M]+, 273 [C17H25N2O]+, 261 [C15H21N2O2] +, 218 [C14H20NO]+, 193 [C10H13N2O2]+, 164 [C10H14NO]+, 148 [C9H10NO]+, 121 [C8H11N]•+, 106 [C8H10]•+, 95 [C5H3O2]+.
{4-[4-({[5-(4-methylphenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (8d)
Light brown liquid; yield: 88%; Molecular formula: C26H24N4O4S; molecular mass: 488g/mol; IR (KBr, υmax cm–1): 3410 (N-H), 3088 (Ar C-H), 2879 (C-H), 1661 (C=O), 1578 (C=C), 1199 (C-O-C), 1111 (C-N-C), 657 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): δ 7.87 (d, J = 6.3 Hz, 2H, H-2'' & H-6''), 7.55 (d, J = 6.4 Hz, 2H, H-3'' & H-5'') 7.46 (br s, 1H, H-5), 7.40 (d, J = 6.4 Hz, 2H, H-2'''' & H-6''''), 7.29 (d, J = 6.3 Hz, 2H, H-3'''' & H-5''''), 7.07-7.05 (m, 1H, H-3), 6.51-6.49 (m, 1H, H-4), 4.52 (s, 2H, H-8''), 3.9 (br s, 4H, H-3' & H-5'), 3.5 (br s, 4H, H-2' & H-6'), 2.42 (s, 3H, H-7''''); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.09 (C-7''), 166.2 (C-5'''), 163.04 (C-2'''), 159.2 (C-6), 147.64 (C-2), 143.96 (C-5), 142.39 (C-4''), 138.13 (C-1''), 134.89 (C-4''''), 129.79 (C-3'' & C-5''), 128.83 (C-2'' & C-6''), 127.64 (C-2'''' & C-6''''), 126.64 (C-3'''' & C-5''''), 120.74 (C-1''''), 117.28 (C-3), 111.55 (C-4), 45.45 (C-2', C-3', C-5' & C-6'), 36.28 (C-8''), 21.64 (C-7''''); EI-MS (m/z): 488 [M]+, 273 [C17H25N2O]+, 261 [C15H21N2O2] +, 218 [C14H20NO]+, 193 [C10H13N2O2]+, 164 [C10H14NO]+, 148 [C9H10NO]+, 121 [C8H11N]•+, 106 [C8H10]•+, 95 [C5H3O2]+.
{4-[4-({[5-(4-hydroxyphenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (8e)
Black brown liquid; yield: 84%; Molecular formula: C25H22N4O5S; molecular mass: 490g/mol; IR (KBr, υmax cm–1): 3416 (N-H), 3089 (Ar C-H), 2870 (C-H), 1661 (C=O), 1574 (C=C), 1191 (C-O-C), 1116 (C-N-C), 649 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): δ 7.93 (d, J = 9.0 Hz, 2H, H-2'''' & H-6''''), 7.80 (d, J = 6.9 Hz, 2H, H-2'' & H-6''), 7.56 (d, J = 7.5 Hz, 2H, H-3'' & H-5'') 7.51 (d, J = 1.9 Hz, 1H, H-5), 7.09 (d, J = 3.7 Hz, 1H, H-3), 6.92-6.90 (d, J = 6.3 Hz, 2H, H-3'''' & H-5''''), 6.51 (dd, J = 1.9, 4.3 Hz, 1H, H-4), 4.52 (s, 2H, H-8''), 3.9 (br s, 4H, H-3' & H-5'), 3.56 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.48 (C-7''), 166.22 (C-5'''), 162.40 (C-2'''), 160.09 (C-4''''), 159.27 (C-6), 147.53 (C-2), 144.07 (C-5), 140.29 (C-4''), 138.30 (C-1''), 130.03 (C-3'' & C-5''), 129.12 (C-1''''), 127.31 (C-2'' & C-6''), 117.45 (C-3), 116.31 (C-2'''' & C-6''''), 115.40 (C-3'''' & C-5''''), 111.59 (C-4), 52.24 (C-2', C-3', C-5' & C-6'), 36.35 (C-8''); EI-MS (m/z): 397 [M]+, 273 [C17H25N2O]+, 261 [C15H21N2O2] +, 218 [C14H20NO]+, 193 [C10H13N2O2]+, 164 [C10H14NO]+, 148 [C9H10NO]+, 121 [C8H11N]•+, 106 [C8H10]•+, 95 [C5H3O2]+.
{4-[4-({[5-(2,4-dichlorophenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (8f)
Light brown liquid; yield: 80%; Molecular formula: C25H20ClN4O4S; molecular mass: 542g/mol; IR (KBr, υmax cm–1): 3405 (N-H), 3082 (Ar C-H), 2880 (R C-H), 1655 (C=O), 1583 (Ar C=C), 1199 (C-O-C), 1110 (C-N-C), 644 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): δ 7.89 (d, J = 8.5 Hz, 2H, H-2'' & H-6''), 7.56-7.55 (m, 2H, H-5'''' & H-6''''), 7.54 (s, 1H, H-3''''), 7.41 (d, J = 6.3 Hz, 2H, H-3'' & H-5'') 7.49 (br s, 1H, H-5), 7.06 (d, J = 2.5 Hz, 1H, H-3), 6.50 (dd, J = 1.7, 3.4 Hz, 1H, H-4), 4.56 (s, 2H, H-8''), 3.85 (br s, 4H, H-3' & H-5'), 3.54 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.04 (C-7''), 164.38 (C-5'''), 163.58 (C-2'''), 159.18 (C-6), 147.62 (C-2), 143.96 (C-5), 138.18 (C-4''), 137.85 (C-4''''), 134.99 (C-1''''), 133.75 (C-6''''), 131.55 (C-1''), 131.24 (C-5''''), 129.58 (C-3'' & C-5''), 129.48 (C-2''''), 127.66 (C-3''''), 127.45 (C-2'' & C-6''), 117.26 (C-3), 111.54 (C-4), 48.50 (C-2', C-3', C-5' & C-6'), 36.24 (C-8''); EI-MS (m/z): 369 [M]+, 245 [C15H21N2O]+, 261 [C15H21N2O2]+, 193 [C10H13N2O2]+, 190 [C12H16NO]+, 136 [C8H10NO]+, 120 [C7H6NO]+, 93 [C6H6N]•+, 78 [C6H6]•+, 95 [C5H3O2]+.
{4-[4-({[5-(3,5-dinitrophenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (8g)
Light brown liquid; yield: 84%; Molecular formula: C25H20N6O8S; molecular mass: 564g/mol; IR (KBr, υmax cm–1): 3415 (N-H), 3062 (Ar C-H), 2880 (R C-H), 1653 (C=O), 1582 (Ar C=C), 1205 (C-O-C), 1107 (C-N-C), 658 (C-S); 1H-NMR (600 MHz, CDCl3, δ in ppm): δ 8.05 (s, 1H, H-4''''), 7.58 (d, J = 6.3 Hz, 2H, H-5'''' & H-6''''), 7.50-7.44 (m, 2H, H-2'' & H-6''), 7.46 (br s, 1H, H-5), 7.27 (d, J = 7.8 Hz, 2H, H-3'' & H-5'') 7.00 (d, J = 2.0 Hz, 1H, H-3), 6.54 (dd, J = 1.6, 3.4 Hz, 1H, H-4), 4.68 (s, 2H, H-8''), 3.80 (br s, 4H, H-3' & H-5'), 3.57 (br s, 4H, H-2' & H-6'); 13C-NMR (150 MHz, CDCl3, δ in ppm): 170.05 (C-7''), 164.31 (C-5'''), 164.12 (C-2'''), 159.20 (C-6), 150.08 (C-3'''' & C-5''''), 147.66 (C-2), 143.94 (C-5), 137.90 (C-4''), 134.24 (C-4''''), 133.04 (C-1''), 131.46 (C-1''''), 130.79 (C-2'''' & C-6''''), 127.69 (C-3'' & C-5''), 122.86 (C-2'' & C-6''), 117.27 (C-3), 111.52 (C-4), 45.48 (C-2', C-3', C-5' & C-6'), 36.24 (C-8''); EI-MS (m/z): 564 [M]+, 496 [C21H16N6O7S]•+, 467 [C20H15N6O6S]+, 385 [C16H9N4O6S]+, 318 [C12H6N4O5S]•+, 246 [C13H14N2O3]•+, 236 [C8H4N4O5]+, 179 [C9H11N2O2]+, 151 [C8H9NO2]+, 118 [C6H2N2O]+, 95 [C5H3O2]+.
Hemolytic activity assay
Bovine blood sample was collected in Ethylene Diamine Tetra Acetic acid (EDTA) that was diluted with saline (0.9% NaCl), and centrifuge at 1000xg for 10 min. The erythrocytes separated diluted in phosphate buffer saline of pH 7.4 and a suspension was made. Add 20µL of synthetic compounds solution (10mg/mL) in 180µL of RBCs suspension and incubate for 30 min at room temperature. Phosphate-buffered saline (PBS) was used as negative control and Triton 100-X was taken as positive control.21,22 The % age of hemolysis was taken as by using formula:
Chemistry
In the present investigation, various novel tri-heterocyclic benzamides were synthesized by the nucleophilic substitution reaction of 2-furoyl-(1-piperazinyl)methanone (1) with 4-(chloromethyl)benzoyl chloride (2) in a basic aqueous medium to get an electrophile {4-[4-(chloromethyl)benzoyl]-1-piperazinyl}(2-furyl) methanone (3). In parallel set of reactions, various substituted-benzoic acids (4a-g) were refluxed with ethanol and conc. sulphuric acid to form respective ethyl substituted-benzoates (5a-g). These esters were further refluxed with N2H4.H2O in methanol solution to acquire substituted-benzohydrazides (6a-g). These hydrazides were cyclized into heterocyclic core by refluxing with CS2 in the presence of KOH and ethanol solvent, giving rise to the formation of various 5-(substituted-phenyl)-1,3,4-oxadiazol-2-thiols (7a-g). The final step in the synthesis was the coupling of electrophile, 3, with nucelophiles, 7a-g, in acetonitrile and potassium carbonate to yield the targeted tri-heterocyclic molecules, 8a-g. Structures of these novel compounds were characterized and confirmed by IR, 1H-NMR, 13C-NMR and EI-MS techniques. (Figure 1) (Table 1).
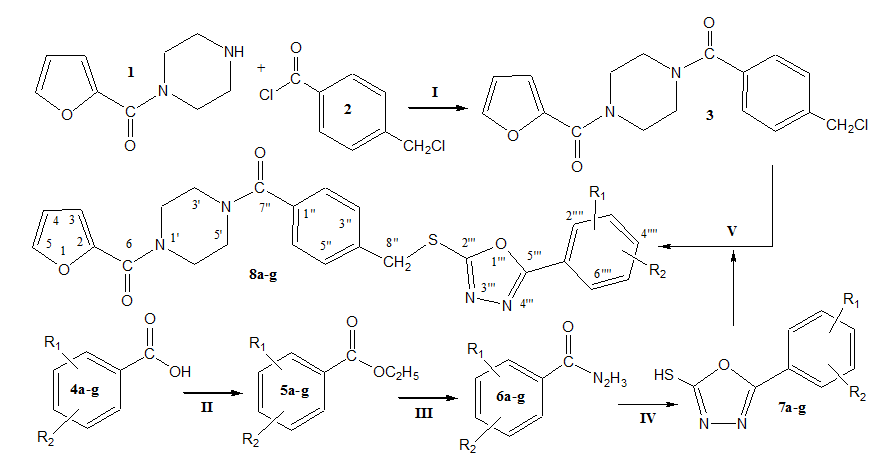
Compound |
R1 |
R2 |
8a |
2-Cl |
H |
8b |
3-NH2 |
H |
8c |
3-NO2 |
H |
8d |
4-CH3 |
H |
8e |
4-OH |
H |
8f |
2-Cl |
4-Cl |
8g |
3-NO2 |
5-NO2 |
Table 1 List of -R1 and -R2 substituents in novel tri-heterocyclic benzamides (8a-g)
One of the compounds is discussed hereby in detail for the expediency of the readers. For example, compound 8d, IR absorption band of aromatic C-H str. appeared at 3088, aliphatic C-H str. at 2879, 1661 (C=O), 1578 (Ar C=C), 1199 (C-O-C), 1111 (C-N-C), 657 (C-S). Its molecular formula was confirmed through EI-MS showing molecular ion peak at m/z 192, 119, 95 corresponding to C26H24N4O4S (Calcd. for 488). The EI-MS spectral data was also in complete agreement for the proposed structure of 8d which was finally confirmed through its 1H-NMR and 13C-NMR spectra.
In 1H-NMR spectrum, signals of methylbenzamide moiety appeared at δ 7.87 (d, J = 6.3 Hz, 2H, H-2'', H-6''), 7.55 (d, J = 6.4 Hz, 2H, H-3'', H-5'') and 4.52 (s, 2H, H-8''). The signals of protons for 4-methylphenyl ring appeared at 7.40 (d, J = 6.4 Hz, 2H, H-2'''', H-6''''), 7.29 (d, J = 6.3 Hz, 2H, H-3'''', H-5'''') and 2.42 (s, 3H, CH3-7''''). Furan ring showed three peaks in aromatic region at δ 7.46 (br.s, 1H, H-5), 7.07-7.05 (m, 1H, H-3) and 6.51-6.49 (m, 1H, H-4). The eight protons of piperazine ring appeared at δ 3.90 (br.s, 4H, CH2-3', CH2-5') and 3.50 (br.s, 4H, CH2-2', CH2-6').
The structure was also thorough supported by its 13C-NMR spectrum. Six signals of 170.09 (C-7''), 142.39 (C-4''), 138.13 (C-1''), 129.79 (C-3'', C-5''), 128.83 (C-2'', C-6'') and 36.28 (C-8'') supported the 4-methylenebenzamide. Two signals of 166.20 (C-5''') and 163.04 (C-2''') corroborated the 1,3,4-oxadiazole ring present in the molecule. Five signals of 159.20 (C-6), 147.64 (C-2), 143.96 (C-5), 117.28 (C-3) and 111.55 (C-4) confirmed the furoyl. Five signals of 134.89 (C-6''''), 127.64 (C-2'''', C-6''''), 126.64 (C-3'''', C-5''''), 120.74 (C-1'''') and 21.64 (C-7'''') confirmed the 4-methylphenyl ring. Piperazine was corroborated by single signal of 45.45 (C-2', C-3', C-5', C-6'). So, on the basis of above discussed cumulative evidences, the structure of 8d was named as {4-[4-({[5-(4-Methylphenyl)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone. Similarly all other synthesized derivatives were characterized by aforesaid spectral techniques.
The %age hemolytic activity and structure-activity relationship (8a-g)
All the synthesized compounds were subjected to hemolytic assay to find out their cytotoxicity profile. Results of percentage hemolysis are shown in Table 2 indicate that all the compounds are nearly nontoxic for membrane of red blood cells. Maximum membrane toxicity was seen by the compound 8e (10.90 %) due to hydroxyl group substitution at para position while minimum was noted in compounds 8a (0.76 %) in which at the o-position occupied by a chloryl group. Overall very mild toxicity was observed for molecules 8d (2.98%), 8c (4.21%), 8g (4.25%), 8b (7.94%) and 8f (10.77%) relative to PBS and Triton-X having % hemolysis of 0.09% and 100% respectively.
Compounds |
Hemolytic Activity (%) |
8a |
0.76 |
8b |
7.94 |
8c |
4.21 |
8d |
2.98 |
8e |
10.9 |
8f |
10.77 |
8g |
4.25 |
Triton-X-100 |
100 |
PBS |
0.09 |
Table 2 Hemolytic activity of synthesized compounds, {4-[4-({[5-(substituted)-1,3,4-oxadiazol-2-yl]sulfanyl}methyl)benzoyl]-1-piperazinyl}(2-furyl)methanone (8a-g).
The anticipated structures of the synthesized tri-heterocyclic molecules, 8a-g, were thoroughly supported by spectroscopic analysis. The hemolytic activity data of these molecules revealed that these have low cytotoxicity and hence might be considered as safe therapeutic agents in drug discovery program.
The Higher Education Commission (HEC) of Pakistan is highly acknowledged by the authors for financial support regarding purchasing of chemicals and spectral study.
There is no conflict of interest.

©2018 Abbasi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.