MOJ
eISSN: 2573-2951


Research Article Volume 4 Issue 2
1Department of Pharmaceutical Technology, University of Dhaka, Bangladesh
2Department of Clinical Pharmacy and Pharmacology, University of Dhaka, Bangladesh
3Department of Pharmacy, University of Dhaka, Bangladesh
4Department of Microbiology, University of Dhaka, Dhaka, Bangladesh
5Department of General Practice, Monash University, Australia
6Department of Pharmaceutical Technology, Jadavpur University, India
Correspondence: Sajal Kumar Saha, Department of General Practice, Monash University, VIC 3169, Melbourne, Australia, Tel +610452639559
Received: October 03, 2017 | Published: November 15, 2017
Citation: Khan SI, Saha SK, Poddar SK, et al. Bioequivalence studies and pharmacokinetic properties of atorvastatin 40mg tablet in healthy bengali subjects. MOJ Bioequiv Availab. 2017;4(2):241-246. DOI: 10.15406/mojbb.2017.04.00064
The present study is aimed to investigate the single-dosing pharmacokinetics of two pharmaceutical formulations of atorvastatin 40 mg tablet, test (T) sample (Stacor®) and reference (R) sample (Lipitor®) in Bengali subjects. An open-label, randomized-sequence, two-way, two-period crossover study was designed with 24 healthy Bengali male volunteers. Plasma samples collected before and over 0.5-72.0h after a single oral dose per subject. Samples were analyzed by solid phase extraction method and a validated LCMS/MS method. The non-compartmental pharmacokinetic model was used to measure pharmacokinetic parameters of peak plasma concentration (Cmax), time to reach the peak (Tmax), elimination half-life (T1/2), elimination constant (kel), and area under the curve to 72h (AUC0-72) and to infinity (AUC0-∞). The mean ± SD values of pharmacokinetic parameters for the test and reference products with minor variation were Cmax: 27.617 ± 2.632ng/mL and 26.166 ± 2.948ng/mL; tmax: 2.479 ± 0.634h and 2.396 ± 0.589h; AUC0-24: 256.799 ± 54.002ng-h/mL and 271.562 ± 52.318h-ng/mL; AUC0-∞: 289.172 ± 56.431ng-h/mL and 309.975 ± 59.594ng-h/mL; kel: 0.055 ± 0.005h-1 and 0.053 ± 0.006h-1; t1/2: 12.591±1.325h and 13.178 ± 1.555h respectively. No significant differences (p > 0.05) for these parameters in the paired t-test and no period or sequence effect was observed in ANOVA analysis. The point estimates (90% CI) of ln-transformed values of Cmax, AUC0-72, and AUC0-∞ were 0.97238 - 1.04110, 0.99296 - 1.00641 and 0.99113 - 1.00255 respectively which fell within the acceptable range of bioequivalence.
Keywords: atorvastatin, pharmacokinetics, bioequivalence, LCMS/MS, bengali subjects
tmax, time to reach the maximum concentration; Cmax, maximum plasma concentration; t1/2, half-life; AUC0-24, area under the plasma time curve up to last quantifiable time; AUC0-∞, area under the plasma time curve up to time infinity; kel, elimination rate constant; NCDs, non-communicable diseases; DGDA, directorate general of drug administration; TPR, total peripheral resistance
There is an emerging importance of bioequivalence and pharmacokinetics analysis of highly prescribed brand drugs to judge their therapeutic effectiveness, toxicity, and quality to ensure therapeutic validity and patient’s safety. Statin classes of lipid-lowering agents are most commonly used treatment intervention for Non-Communicable Diseases (NCDs) like diabetes, obesity, hypertension, and dyslipidemia.1 Atorvastatin is an FDA approved synthetic lipid reducing agent under statin class. This drug, also known as 2-(4-fluorophenyl) - βR, δR-dihydroxy-5-(1-methyl ethyl)-3-phenyl-4(phenylamino) carbonyl]-1H-pyrrole-1-heptanoic acid (Figure 1), is a second generation 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor.2 This synthetic HMG-CoA reductase inhibitor significantly lowers total cholesterol, low-density lipoprotein cholesterol, and plasma triglycerides in clinical studies.3 According to IMS Health, Lipitor, the pioneer brand of atorvastatin is the world's one of the biggest selling prescription medicines with cumulative sales in excess of $130 billion, but most of the principal brands are now prescribed worldwide as far cheaper generic medicines. In Bangladesh, about 50 atorvastatin brands with different strengths and dosage forms are available and their average sales volume is nearly 1.17 crore taka monthly in the national local market according to Bangladesh Directorate General of Drug Administration (DGDA). This indicates widespread prescribing of this cholesterol-lowering drug in Bangladesh. However, there is no bioequivalence data for any of existing 50 brands of atorvastatin.
Dose-dependent lowering of total cholesterol, LDL cholesterol and triglyceride levels have been noticed after oral administration of atorvastatin to patients with hypercholesterolemia and to patients who have hypertriglyceridemia.4,5 The usual starting dose is 10mg atorvastatin daily. The dose should be adjusted in accordance with plasma lipid levels and can be increased to up to 80mg daily.6,7 Pharmacokinetically, atorvastatin has rapid absorption after oral administration due to its high membrane permeability and solubility and maximum plasma concentrations are obtained within 1 to 3 hour.8 Multiple daily doses of 2.5 to 80mg atorvastatin produce steady-state maximum plasma concentrations (Cmax) of 1.95 to 252µg/L within 2 to 4 hours after administration and area under the plasma concentration-time curve (AUC) values of 25.2 to 1293µg/L.9
The bioavailability of atorvastatin is only ~14% because of extensive pre-systemic extraction in the gastrointestinal tract and/or liver.7,10 This drug is ≥ 98% protein-bound in plasma. The mean volume of distribution of atorvastatin is reported to be 381L after intravenous infusion of 5mg which clearly indicates its extensive binding to peripheral tissues.10 This drug has a mean elimination half-life of about 14 hours, but due to the presence of active metabolites, the half-life of HMG-CoA reductase inhibition becomes 20 to 30 hours. The active acid form of atorvastatin is mainly used for an administration which undergoes extensive first-pass metabolism, predominantly by cytochrome P450 3A4 (CYP3A4) in the liver.7 Less than 2% of the parent drug and metabolites follow renal excretion.11 Like other HMG-CoA reductase inhibitors, the gastrointestinal disturbance is the most frequently reported adverse event associated with atorvastatin.12 It depends on pharmacokinetic variation, subject variation and even metabolic rate from the context of racial and ethnic variation. Consequently, its pharmacokinetics should be well established as country and population specific to predict the toxicity of atorvastatin and to optimize dose, which would likely to ensure patient safety.
In fact, tolerability and bioequivalence of different preparations containing same active ingredient have gained considerable importance over the past few years because of increasing generic substitution and hence cheaper price. However, as the development of generic drugs is mainly based on pharmacological properties of the original brand, one would expect the same quality and almost similar effects like that of the original brand.13 There is insufficient pharmacokinetics and bioequivalence study on widely used drugs in Bangladesh and atorvastatin is one of them. Similarly, no pharmacokinetics and bioequivalence evaluation of atorvastatin 40mg tablet have been established yet in Bangladesh despite enormous prescribing of atorvastatin from primary to tertiary care facilities. Therefore, the aim of this study was to evaluate pharmacokinetics and bioequivalence of two pharmaceutical products of atorvastatin; Stacor® (T) and Lipitor® (R) tablet in healthy Bengali subjects in order to establish quality, safety and even tolerability of this generic drug.
Drugs and reagents
Two commercially available brands of 40mg atorvastatin, Stacor® tablet (T) (Batch No: MH 51, DAR No: 225-291-25 manufactured at 08/2011 by Unimed-Unihealth Pharmaceutical Ltd, Dhaka, Bangladesh) and Lipitor® tablet (R) (Batch No: 0744010 U manufactured at 09/ 2011 by Pfizer Ireland Pharmaceuticals, Dublin, Ireland) were obtained. Preparation of deionized water was done using Milli-Q system (Continental Water Systems, El Paso, TX, U.S.A.). HPLC grade formic acid and methanol, and analytical grade ethyl acetate were procured from Merck, India.
Subjects
Twenty-four healthy Bengali male volunteers participated in the study (Table 1). No abnormalities were detected in the routine laboratory (hematological and biochemical) and clinical tests that were pre-requisite as inclusion criteria. All the subjects avoided alcohol consumption, drug abuse, concomitant medication use and smoking during the period of the study.
Sequence Group |
||||
Parameter |
RT (n = 12) |
TR (n = 12) |
Total (N = 24) |
P-Value |
Age, y |
26.91 (2.34) |
26.23 (2.04) |
26.46 (3.88) |
0.786 |
Height, cm |
164.66 (4.30) |
163.29 (4.54) |
163.38 (4.43) |
0.376 |
Weight, kg |
59.55 (5.43) |
58.64 ( 5.35) |
59.25 (5.3) |
0.645 |
Body Mass Index, kg/m2 |
21.43 (1.33) |
21.21 (1.65) |
21.36 (1.53) |
0.854 |
Male Sex |
12 |
12 |
24 |
|
Smoke (Yes/No) |
4/8 |
5/7 |
9/15 |
0.201 |
Alcohol (Yes/No) |
0/12 |
0/12 |
0/24 |
0.986 |
Caffeine (Yes/No) |
6/6 |
5/7 |
11/13 |
0.658 |
Table 1 Demographic characteristics of study subjects.
*Values are given as mean (SD) or number.
RT: Reference tablet first then test tablet administered; TR: Test tablet first then reference tablet administered.
P< 0.05 indicated significant difference among two sequence group RT and TR.
The protocol of the whole research was approved by the clinical review committee, Faculty of Pharmacy, University of Dhaka. Demographic data were collected from every participant who gave written consent after reading the protocol.
Ethical review and consent procedure
Guidelines, as drawn up by the Bangladesh Medical Research Council (BMRC), were followed regarding the treatment of human volunteers in the study. These guidelines were in accordance with the U. S. Code of Federal Regulations (Title 21, Part 56), the Declarations of Helsinki and the Canadian MRC Guidelines. The study was initiated after approval of the protocol, ethical clearance and the informed consent form by BMRC, Bangladesh. The ethical clearance number was BMRC/NERC/DO 2013-2016/1020 date 08.09.2015.
Confidentiality
Every prospective candidate was given a full explanation of the study. Once all the necessary information was provided to them and the physician in charge was confident enough that he understood the implications of participation in the study, the subject was asked to sign the consent form. It was agreed that the protocol information and the results of the study would remain undisclosed to others, without written authorization from the principal investigator.
An open-label, randomized-sequence, two-way, two-period crossover study with single dose was designed with 24 healthy Bengali male volunteers. All the volunteers participated in two dosing sessions with a respite of 15 days washout period. As per the randomization code at a fixed time, volunteers received either the test preparations or reference preparations as a single dose in each dosing session on that particular study day. Volunteers were given code numbers by the bio-statistician and were allocated to the treatment in accordance with the randomization code. Neither the personnel in charge of the determination of plasma levels nor the physician and nursing staff in charge of the clinical aspects was informed of the sequence of administration.
After overnight fasting for 10 hours, all the volunteers assembled in the medical center, the University of Dhaka at 6.00 a.m. on the study day of each session. Total peripheral resistance (TPR), blood pressure (BP) of every volunteer was recorded and an indwelling intravenous catheter was inserted in the suitable vein with strict aseptic precautions for blood collection. A total of 14 blood samples were collected at 0 h (before drug administration) and 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 8.0, 12.0, 24.0, 48.0, and 72.0 h (after drug administration) in the EDTA containing test tubes over 72hrs period of each two sessions. Following ingestion of the drug, breakfast, lunch, and dinner were provided after 3hrs, 6hrs, and 13hrs respectively. Except for strenuous exercise, volunteers were permitted to do normal activities on the study days. Collected blood samples were centrifuged immediately at a speed of 3000rpm for 15 minutes and separated plasma was stored frozen at -800C with appropriate labeling of volunteer code no., study date and collection time. Monitoring of abnormal signs and symptoms was carried out during the study period and the following one week of the study period.
Sample preparation
1.0mL of plasma sample was mixed with 100μL of 1:4 (v/v) methanol: acetate buffer (pH 4.6, 1:4 v/v) mixture followed by further mixing with 5.0mL of dichloromethane: acetonitrile (4:1v/v). The blend was vortex mixed for 30 seconds. Again after centrifugation at 4500rpm for 10min, 4mL of the organic phase was separated and evaporated under a nitrogen stream. After dissolving the residue in the mobile phase, 25.0μL of it was injected into LCMS system.
Identification and quantification of the atorvastatin by using LCMS/MS (Shimadzu Prominence, Kyoto, Japan) detection were done for determination of the pharmacokinetic parameters. The method, used for analysis of atorvastatin in a plasma containing an anticoagulant, was validated.
Chromatographic analysis
Atorvastatin and internal standard rosuvastatin plasma levels were determined by Shimadzu Prominence (Kyoto, Japan), an LC-MS system which consists of Integrated System Shimadzu Controller SIL-20AC, prominence, autosampler with two pumps Shimadzu LC-20AD, prominence, and liquid chromatography; Pump A Model: LC-20AD , Pump B Model: LC-20AD. Pumping models were of binary flow. Model SIL-20AC was used as the auto-sampler in which rinsing volume, needle stroke, rinsing speed, sampling speed, purge time, rinse dip time and the cooler temperature was fixed at 200uL, 52mm, 35µL/sec, 15.0uL/sec, 25.0min, 0 sec and 150C respectively. Separation of compounds was carried out by column (C19, 50 x 4.5mm, 5µ particle size) (Phenomenox, Gemini, Torrance, USA) eluted with 9:1(v/v) of MeOH: H2O (Containing 1% formic acid) at room temperature having a flow rate of 0.5000mL/min.
Pharmacokinetic analysis
Plasma drug concentrations for both test and reference product at specified time points were used in pharmacokinetic calculations of the study. For estimating pharmacokinetic parameters, the dataset was prepared by using program kinetic (version 4.4, Adept Scientific, UK) followed by the non-compartmental method of analysis. The pharmacokinetic parameters, included in the study protocol, comprised: time to reach the maximum concentration (tmax), maximum plasma concentration (Cmax), half-life (t1/2), area under the plasma time curve up to last quantifiable time (AUC0-24), area under the plasma time curve up to time infinity (AUC0-∞), elimination rate constant (kel) and ratio Cmax /AUC0-∞.
Recovery experiment
Determination of the percentage recoveries was done by measuring the peak areas of the drug from the prepared plasma low, medium and high-quality control samples. The peak areas of the plasma low, medium and high-quality control samples were then compared to the absolute peak area of the un-extracted standards containing the same concentrations of the atorvastatin. The results are presented in Table 2. Recovery after extraction was 81.82% - 85.77%.
Inj No. |
Aqueous |
In Plasma |
||||
QCL 3ng/mL |
QCM 30ng/mL |
QCH 40ng/mL |
QCL 3ng/mL |
QCM 30ng/mL |
QCH 40ng/mL |
|
1 |
2.839 |
27.359 |
39.864 |
2.697 |
25.554 |
34.229 |
2 |
2.708 |
28.588 |
36.604 |
2.194 |
24.3 |
35.44 |
3 |
2.912 |
26.936 |
39.779 |
1.976 |
21.961 |
31.615 |
4 |
2.707 |
29.444 |
37.242 |
2.17 |
23.752 |
32.536 |
5 |
2.97 |
28.742 |
38.523 |
2.529 |
22.49 |
30.878 |
Mean |
2.827 |
28.214 |
38.403 |
2.313 |
23.612 |
32.94 |
% Recovery |
81.82 |
83.69 |
85.77 |
|||
Table 2 Absolute recovery of analyse from plasma drug samples.
The primary variables Cmax, AUC0-t, and AUC0-∞ were taken into consideration to evaluate the bioequivalence between the test and reference formulations. Two-way ANOVA with crossover design (2x2) was used to determine the effects of formulation, period, sequence, and subjects on log-transformed Cmax, AUC0-t, and AUC0-∞. The ratios of the log-transformed Cmax, AUC0-t, and AUC0-∞ values were calculated for both formulations and 90% CIs were obtained. The two formulations were considered bioequivalent based on 90% CI of the test/reference ratios of AUC within 0.80 to 1.25.
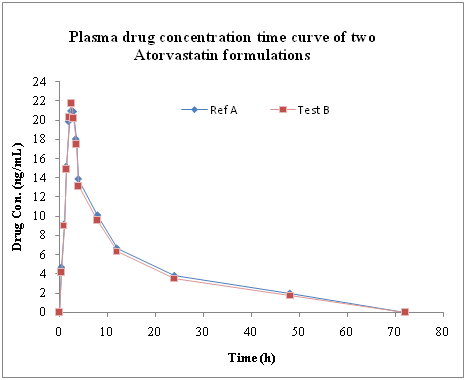
The mean plasma concentrations of atorvastatin observed in the 24 healthy volunteers studied with two oral pharmaceutical formulations: Lipitor® and Stacor® were shown in Figure 2. A total of 4 mild adverse events including 1 abdominal discomfort and 3 venipuncture syncope were reported by three subjects (6.5%). These were not considered to be associated with the administration of the atorvastatin. There is no significant change in serum pharmacokinetic parameters like Tmax, Cmax, t1/2, AUC0-24, AUC0-∞, kel of 24 volunteers with the Test and Reference preparation (Table 3). The test preparation of tablet Stacor® 40 produced the maximum plasma concentration 27.617 ± 2.632ng/mL (Cmax) at the time 2.479 ± 0.634 hour (tmax), whereas, the reference preparation, tablet Lipitor® 40mg, produced Cmax 26.166 ± 2.948ng/mL at the time 2.396 ± 0.589 hour (tmax). Atorvastatin kinetics exhibited a similar pattern followed by administration of either formulation. Inter-individual variation in plasma concentrations of atorvastatin was small (Table 4). P- values of the pharmacokinetic parameters were found out to be: 0.257 for Cmax; 0.657 for tmax; 0.879 for AUC0-24; 0.856 for AUC0-∞; 0.763 for MRT; 0.451 for AUMC0-24; 0.485 for AUMC0-∞; 0.640 for kel. These high P-values indicate a lack of significant difference in each biochemical parameter. Based on a comparison of the AUC0-t for atorvastatin after single dose administration, the relative bioavailability of the test preparation was 94.56% of that of the reference preparation. The subject, period and treatment were applied to the AUC0-t, ln AUC0-t, Cmax and in Cmax values. The 90% confidence interval of ratios of test and reference preparation for Cmax, AUC0-24 and AUC0-∞ values are stated in Table 5. No statistically significant difference was found for the treatment values of AUC0-t, ln AUC0-t, Cmax and ln Cmax.
Pharmacokinetic Parameters |
Mean |
Geometric Mean |
CV (%) |
SD |
Max |
Min |
Reference Formulation (R) |
||||||
Cmax (ng/mL) |
26.166 |
26.001 |
11.268 |
2.948 |
32.163 |
19.106 |
Tmax (h) |
2.396 |
2.325 |
24.6 |
0.589 |
3.5 |
1.5 |
AUC0-24 (h.ng/mL) |
271.562 |
266.69 |
19.266 |
52.318 |
379.243 |
156.634 |
AUC0-∞ (h.ng/mL) |
309.975 |
304.519 |
19.226 |
59.594 |
460.19 |
179.305 |
Kel |
0.053 |
0.053 |
11.327 |
0.006 |
0.063 |
0.043 |
t1/2 (h) |
13.178 |
13.0928 |
11.907 |
1.555 |
16.159 |
10.955 |
Test Formulation (T) |
||||||
Cmax (ng/mL) |
27.617 |
27.495 |
9.53 |
2.632 |
32.373 |
22.915 |
Tmax (h) |
2.479 |
2.4 |
25.566 |
0.634 |
3.5 |
1.5 |
AUC0-24 (h.ng/mL) |
256.799 |
251.503 |
21.029 |
54.002 |
387.678 |
153.488 |
AUC0-∞ (h.ng/mL) |
289.172 |
283.948 |
19.515 |
56.431 |
415.642 |
175.088 |
Kel |
0.055 |
0.055 |
9.664 |
0.005 |
0.067 |
0.044 |
t1/2 (h) |
12.591 |
12.524 |
11.915 |
1.325 |
15.575 |
10.292 |
Table 3 Plasma pharmacokinetic parameters of 24 volunteers with the test and reference formulations.
Pharmacokinetic Parameters |
Sources of Variation |
|||
Formulation |
Period |
Sequence |
Subject |
|
Cmax (ng/mL) |
0.18 |
0.57 |
0.36 |
˂0.01 |
tmax (h) |
0.47 |
0.67 |
0.54 |
0.07 |
AUC0-24 (h.ng/mL) |
0.78 |
0.78 |
0.46 |
˂0.01 |
AUC0-∞ (h.ng/mL) |
0.76 |
0.89 |
0.85 |
˂0.01 |
Kel |
0.64 |
0.34 |
0.71 |
0.13 |
t1/2 (h) |
0.76 |
0.42 |
0.83 |
0.17 |
Table 4 P-values for sources of variations in plasma pharmacokinetic parameters obtained from Analysis of Variance (ANOVA).
Untransformed Data |
ln-Transformed Data |
|
Cmax |
0.89461 - 1.15268 |
0.97238 - 1.04110 |
AUC0-t |
0.95754 - 1.03991 |
0.99296 - 1.00641 |
AUC0-∞ |
0.94615 - 1.01599 |
0.99113 - 1.00255 |
Table 5 90% confidence interval of the ratios of the Test and Reference preparation.
The Food and Drug Administration (FDA) highlights the same quality, effectiveness, and performance of generic drugs as brand-name drugs and it does not allow the difference in the effectiveness of generic drug product about 45 percent. A recent FDA’s review of 2,070 human studies between 1996 and 2007 stated that average difference in absorption between the generic and the brand was 3.5 percent.14 Another study compared cardiovascular generic drugs to their brand name counterparts taking 38 published clinical trial results and found no evidence that brand-name cardiovascular drugs worked any better than generic cardiovascular drugs.15 Statin drugs are one of the drug classes among them. However, these trials are rare among low and middle-income countries although many of these countries have huge drug market. Bangladesh is one of them.
To ensure the clinical effectiveness and safety of the generic product to that of innovator brand and due to the unsuitability of repetition of clinical studies for generic products, pharmacokinetics and bioequivalence studies are considered as ‘second to none’. Determination of parent drug release from dosage forms over metabolites is a general recommendation for bioequivalence studies from US FDA guidelines.16 The considerable variability between individuals and substantial differences between the two formulations within each individual are two striking observations under important consideration in any comparative pharmacokinetics study. Considerable interindividual variability, particularly in Cmax, Tmax, and AUC is evidenced for many drugs. This variability might be due to genetic polymorphism of the cytochrome P450 (CYP) isoform CYP2C19.17-20 Additionally, demographic data of subjects; age, weight, height and concomitant medication could add to the high inter-individual variability.
In the present study, atorvastatin 40mg tablet was detected in plasma from 0.5 hours to 48.0 hours in the reference and test preparations after administration. Peak plasma levels of atorvastatin 40mg for both the preparations were achieved between 2.0 and 3.5 hours (Figure 2). The mean peak plasma levels of reference formulation ranged between 19.106-32.163ng/mL compared to test preparation ranged between 22.915-32.373ng/mL (Table 3).The ratios of Cmax between these two formulations were ranged between 97%-104% (Table 5). Similarly, other main pharmacokinetic parameters Tmax and AUC for both formulations were compared by analysis of variance and no statistically significant difference was observed (Table 4). In fasting state, Cmax, tmax and AUC0-t values were comparable for the reference and the test preparation. Moreover, 90% confidence limits of ratios for Cmax, AUC0-72 and AUC0-∞ were found to be within the limits of bioequivalence acceptance ranges. The Cmax, AUC0-24 and AUC0-∞ ratios were 0.97238 - 1.04110, 0.99296 - 1.00641 and 0.99113 - 1.00255 respectively, which falls within the accepted limit to that of the reference preparation (i.e. 0.8 - 1.2). In this study, we selected these limits of acceptance, based on the variability of atorvastatin pharmacokinetics and to the fact that atorvastatin shows double peaks or major shouldering characteristics. It has been recommended that, in the case of drugs with a wide variability in absorption, these limits are adequate and are accepted in Europe.21,22 Based on these study derived results, it can be inferred that the tested formulation of atorvastatin is bioequivalent with innovator formulation.
However, this study had some limitations. Firstly, the study was open labeled which might not able to address tolerability of two formulations. Secondly, as the study was conducted solely on healthy Bengali subjects who are free from hypercholesterolemia and hypertriglyceridemia, pharmacokinetics might be different for patients and/or other targeted populations. Thirdly, the study was limited only to male volunteers. Thus, gender variation in the pharmacokinetics of atorvastatin in Bengali subjects was not reflected in the study. Fourthly, the study didn’t observe the effect of food on pharmacokinetics. Further research regarding gender variation and effect of food on the pharmacokinetics of atorvastatin on Bengali population is recommended.
The study found that the reference and test formulations of atorvastatin 40mg tablet are pharmacokinetically safe and met the regulatory benchmark for bioequivalence in healthy Bengali subjects. The test product Stacor® may be supplanted for reference product Lipitor®.
There was no fund for this research work. However, authors contributed to bear part of expenditures and department of pharmaceutical technology, University of Dhaka, Bangladesh and Jadavpur University, Kolkata provided reagents and equipment support to carry out this work.
The authors are grateful to bioequivalence center, Jadavpur, Kolkata for quantitative analysis and to all the volunteers who took part in the study as study subjects.
The author declares no conflict of interest.

©2017 Khan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.