MOJ
eISSN: 2573-2951


Research Article Volume 5 Issue 5
1Bioterio–INDICASAT AIP, Panamá
2Centro de Biología Celular y Molecular de Enfermedades, Instituto de Investigaciones Científicas y Servicios de Alta Tecnología-INDICASAT AIP, Panamá
Correspondence: Rosa De Jesus, BioterioINDICASAT AIP, Panamá, Tel 0507-63541171
Received: June 26, 2018 | Published: October 4, 2018
Citation: Jesus RD, Spadafora C. Evaluation of the malaria development in a group of CB6F1 MICE, produced in the laboratory animal facilities of indicasat AIP. MOJ Bioequiv Availab. 2018;5(5):264-268. DOI: 10.15406/mojbb.2018.05.00112
The purpose of this assay was to characterize the infection by Plasmodium berghei of three groups of inbred mice to determine which group is more appropriate to use as a biomodel for testing ethnobotanical anti-malarial compounds. A group of mice, CB6F1 (BALB/c x C57BL/6), and two inbred strains of mice, C57BL/6 and BALB/c, were parasitized with P. berghei (ANKA) and were evaluated as to average weight, behavioral parameters and integrity of the Brain Blood Barrier. These data were correlated to their parasitaemia. In reference to the average weight, all of the animals in the three groups lost weight as the infection progressed. This decrease did not present significant differences between the individual animals of the group (p=0.8841). When correlating the four stages to the evolution of malaria, Stages III and IV of disease progression correlated to the manifestation of cerebral malaria, verified by the presence of injury to the Brain Blood Barrier. Thirty-seven percent of the CB6F1 mice showed signs of Stage IV, and 63% showed signs of Stage III, both with a median parasitaemia of 24±2,7%. One hundred percent of C57BL/6 mice presented Stage III, with a median parasitaemia of 28%, while the BALB/c showed no clinical manifestations of cerebral malaria, in spite of parasitaemias as high as 60% at the time of death. The tests revealed that strain C57BL/6 is more appropriate to use as a biomodel for testing ethnobotanical anti-malarial compounds.
Keywords: mice, cerebral malaria, C57BL/6, BALB/c, CB6F1, berghei
Malaria infection is caused by protozoan parasites of the genus Plasmodium. According to the latest report of the World Health Organization, this disease is one of the top 10 causes of death in low-income countries and the foremost public health problem worldwide, with more than 212 million new cases and 429,000 deaths per year. The report also states that the disease is endemic to 91 countries, including the countries of the American continent with the exception of USA, Costa Rica and Argentina.1
Cerebral malaria (CM) is the most severe and often fatal complication of the disease, mainly affecting children under the age of 5 in sub-Saharan Africa.2 Attempts to get an early diagnosis of CM are not generally successful, and even with the available treatments 15–20% of the patients die, while 10–15% of those cured will suffer long-term neurological deficits.3,4
Experimental malaria studies such as physiopathological assessment, molecular mechanisms, and tests of pharmaceutical products5–8 have been carried out in different murine models. Other studies on the developmental phases of the parasite and their clinical manifestations have allowed the classification of the mice as susceptible or non-susceptible models. The condition of susceptibility targeted this study relates to cerebral malaria pathology.9
The clinical signs of CM in susceptible mice are: ataxia, respiratory distress, development of neurological signs, coma and death, which appear around 8-10 days post infection. Histopathological analysis reveals vascular clogging, petechial hemorrhages and leukocyte sequestration with a relatively low sequestration of infected red blood cells.10, 11
The different neurological CM signs that occur in humans are difficult to identify in mice.12 The association of physiopathological changes occurring early during infection with outcome, which is crucial for the understanding of CM pathogenesis, is inhibited by a number of factors related to the experimental model.6 The neurological signs in mice (ataxia, convulsions, roll over, paralysis, coma) develop only a few hours before death.11 Moreover, different factors such as genetic background, age, inoculum size, parasitaemia course and clonal variations of the parasite also interfere with the incidence of CM in mice.9 For this reason, it is important to identify the different stages of the disease and their manifestations in order to characterize the infection in different strains of mice.
Different authors have used the SHIRPA protocol (SmithKline Beecham, Harwell, Imperial College, and Royal London Hospital) to evaluate behavior and development of neurological signs in mice, comparing the behavior of Plasmodium parasitized mice with the neurological signs that occur in CM. Strains of mice have been characterized using this protocol, revealing differences between these.13, 14, 15, 9, 12, 16
Similarly, the cerebral oedema pathology is involved in CM, accounting for the permeability changes in the Brain Blood Barrier (BBB) in susceptible mice.17 Evans Blue solution has been used to evaluate BBB injuries and therefore the clinical pathology of the disease in mice, with the intensity of the blue coloration in the cerebral tissue signaling a faulty permeation of the barrier. This is possible due to the afinity of the stain for plasma albumin which efficiently crosses the BBB.18, 19, 12
The objective of the study was to evaluate and compare certain phenotypic manifestations such as average weight, behavioral characteristics and certain neurological signs implicated in CM caused by Plasmodium berghei (ANKA), and to correlate them with parasitaemia and BBB integrity in CB6F1 mice groups and compare these parameters with C57BL/6 mice, already characterized as susceptible to CM, and with BALB/c mice, characterized as non-susceptible. The murine models thus characterized through this study will be validated for their use in the evaluation of antimalarial ethnobotanical compounds in the INDICASAT AIP animal facilities of Panamá.
Sixty (60) female mice were used, twenty (20) each for three different genetic groups: C57BL/6, BALB/c and CB6F1. Two assay groups were formed: one (1) parasitized group and one (1) healthy group which was used as the control.
The mice were 7 to 8 weeks old. The animals were housed in conventional facilities at the INDICASAT AIP animal facilities in ventilated racks and received a standard sterilized diet (LabDiet, USA) and water ad libitum, with sanitary barriers: sterilization of bedding and food, filtered water and change of clothes of the personnel. The rooms were set up with cycles of 12 hours of light and 12 hours of dark.
Mice were infected with Plasmodium berghei (kindly donated by Patricia Llanes of the Center for Cellular and Molecular Biology of Diseases, INDICASAT AIP), through intraperitoneal injection of 5 x 106 parasitized red blood cells (pRBC). PRBCs were isolated from the blood of infected BALB/c mice at 30 to 40% parasitaemia obtained from intracardiac blood of anesthetized mice with ketamine/xylacine 120/10 mg/kg weight. The control group was injected with an equivalent volume of healthy erythrocytes from the same mouse strain.
Research complied with all relevant ethical guidelines and institutional policies of INDICASAT AIP for the use of laboratory animals, and the approval of the intrainstitutional committee for the care and use of laboratory animals, with registration CICUA-16-009.
Evaluation of clinical parameters
Behavioral parameters |
Score |
|||
|
3 |
2 |
1 |
0 |
Locomotor activity |
walk quickly |
walk slowly |
walk and stops |
Does no walk |
Straightening reflex* |
return immediately |
after of 2 attempts |
after of 5 attempts |
remains on the back |
Pelvic elevation |
- |
on its 4 tips |
on side |
markedly fainted |
Elevated tail |
- |
elevated |
little elevated |
flattened |
Exhaust reaction to finger pressure |
- |
response |
attempts response |
no response |
Tremor |
- |
- |
absent |
present |
Piloerection |
- |
- |
absent |
present |
Deviation of head |
- |
- |
absent |
present |
Table 1 Behavioral parameters and scores for determining the presence of Cerebral Malaria
*Wilson et al.,27*
Other conditions, such as tremor, piloerection and deviation of head, were evaluated as present (1) or absent (0).
Parasitaemia percentages were compared with certain behavioral parameters used in the SHIRPA protocols.14, 20, 21 Briefly, these are: locomotor activity, straightening reflex, pelvic elevation, elevated tail, exhaust reaction to finger pressure, tremor, piloerection, and deviation of head. Another important parameter evaluated was the permeability of the Blood-Brain Barrier (BBB), as described above.12 The evaluations took place daily. The behavioral parameters were measured placing the mice in an open field away from the accommodation cage, for a period of 5 minutes/animal/day. Timing was controlled by means of a stopwatch. The open field was cleaned with 95% ethanol/5% water after each mouse exited the space, and left to dry before the next one entered.
The behavioral parameters were compared with neurological alterations associated with Cerebral Malaria 10,12 and compared between parasitized mice and healthy mice. The Cerebral Malaria signs presented in four stages,12 as detailed in Table 1: Stage I (asymptomatic), in which parasitized mice have a behavior similar to healthy mice (controls), presenting a high total score of 21 (3- locomotor activity, 3- straightening reflex, 2-pelvic elevation, 2- elevated tail, 2- exhaust reaction to finger pressure, 1- tremor, 1- piloerection, 1- deviation of head) and no neurological alterations; Stage II, in which mice begin presenting neurological alterations (2- locomotor activity, 3- straightening reflex, 1- pelvic elevation, 1- elevated tail, 2- exhaust reaction to finger pressure, 1- tremor, 1-piloerection, 1- deviation of head), with a total score lower than 12. Stage III, showing clear health deterioration(1- locomotor activity, 3- straightening reflex, 1- pelvic elevation, 0- elevated tail, 1- exhaust reaction to finger pressure, 0- tremor, 0-piloerection, 0- deviation of head), with a score of 6. Stage IV, where clinical signs of cerebral malaria are evident and the behavioral parameters present scores of zero (0)(0- locomotor activity, 0- straightening reflex, 0- pelvic elevation, 0- elevated tail, 0- exhaust reaction to finger pressure, 0- tremor, 0-piloerection, 0- deviation of head).
The Brain Blood Barrier (BBB) was evaluated when the parasitaemia percentage was at or above 24% for all CB6F1 and C57BL/6 mice and at or above 60% for BALB/c mice. The animals were administered 200 μL of 2% Evans Blue solution intraperitoneally and one hour later the animals were sacrificed with an injection of ketamine/xylazine mixture (brand: (KEPRO – HOLLAND) at a dosage of 120/10mg/kg of weight. The brain tissue was observed and the degree of blue coloration was determined.12
The parameters evaluated with the SHIRPA protocol and the presence of BBB injury as a method of evaluating the evolution of cerebral malaria with P. berghei in three groups of mice produced in the INDICASAT AIP facilities, were selected for their high reliability and reproducibility as well as their affordability, requiring no use of high-end equipment.3
Statistic analysis
Parasitaemia percentages and weight averages were presented as means of the individual numbers with standard errors of the mean (SEM). The normality of the data was calculated using the Shapiro-Wilk test with a significance set at p>0.05. The comparison between data was made by Kruskal-Wallis One Way AOV-AOV Table (Statistix 10 Programm) using the observations made on day 7 after infection.22
Murine models BALB/c and C57BL/6 have been used in malaria assays and are susceptible and non-susceptible to developing cerebral malaria, respectively.2, 23 Nonetheless, these models present important differences and contradictions [21,10], such as genetic background, age or inoculum size needed for infection, with regard to neurological signs of cerebral malaria or the developmental course of the parasite, among others.
In our study, the parasitaemia evolution from the first day post inoculation presented differences among the three groups of mice used: C57BL/6, CB6F1 and BALB/c. The C57BL/6 and CB6F1 mice presented a parasitaemia with constant increases across the observation period, reaching a percentage of 28±2.3% and 24±1.5% at day seven post infection, respectively, while the BALB/c mice parasitaemia fluctuated, showing increases and decreases across the 11 days that the study spanned, with an average of 26.5±2.1% (Figure 1). Statistical analysis of parasitaemia in the three groups, done up to the seventh day because by day eight most of the C57BL/6 mice had died, did not present significant differences (p=0.895).
Similar results were reported in assays with C57BL/6 mice, however,24, 12, 25 parasitaemias higher than 80% have also been reported for this strain by the 14th day post infection.26 As for the BALB/c mice parasitaemia, distinct results have been reported, with increases and decreases throughout the course of infection, even reporting hyperparasitaemias.27
Figure 1 Parasitaemia of infected mice. Three strains of mice were inoculated with P. berghei (ANKA) and the course of parasitaemia was monitored daily for up to eleven days.
In relation to average weight, all the mice lost weight except the controls. C57BL/6, CB6F1 and BALB/c mice lost an average of 2.90%, 2.62% and 1.46%, respectively, with no significant differences among them (p=0.8841). This is in accordance with similar reports published in the past.23
The mice of the CB6F1 group presented weight and parasitaemia percentages with values that are intermediate between the other two groups of the assay.
Figure 2 presents some images of what was observed in the three groups of mice during the course of the infection, when the behavioral parameters were studied and correlated to Cerebral Malaria development.
Images a and b present the behavior of the BALB/c mice group. In image (a), a mouse is presented, 24 h post inoculation and with a parasitaemia of 1%; its behavior was similar to that of the control. Image (b) shows a BALB/c mouse eleven (11) days post inoculation, with a parasitaemia of 60%, and presenting the following characteristics: no locomotor activity (score 2), piloerection (P) and flattened tail (score 0), however, it did not present Cerebral Malaria signs, and in the evaluation of the Blood Brain Barrier did not show injury (images 2a, 2b), therefore, they did not develop Cerebral Malaria despite the high parasitaemias they evidenced. As for C57BL/6 mice, in image (2c) a mouse with Stage 1 phenotype behavior (asymptomatic) can be seen, similar to the behavior of the control mice, despite having a parasitaemia of 3%. Images 2d and 2e represent the altered behavior displayed by 80% of the mice, such as: no locomotor activity (score 0), flattened tail (score 0), pelvic elevation markedly fainted (score 0), piloerection (P), exhaust reaction to finger pressure (score 0), deviation of head (P), and tremor (1); all of these signs indicated that the mice were in Stage III; moreover, they presented Blood Brain Barrier injury (Figure 3.3b). However, 20% of the group of C57BL/6 mice presented different scores for parameters such as: exhaust reaction to finger pressure (score 1), deviation of head (A), and tremor (2), and did not present an injured blood brain barrier (Figure 3.3a) despite having a parasitaemia of 28%, correlating with an absence of neurological signs. Similar reports were presented by Martins et al. 9 and Martínez et al. 12
In images 2f to 2i, the evolution of behavior from Stage I to Stage IV can be observed in the group of CB6F1 mice. These mice, conversely, did present signs of Cerebral Malaria, manifested as straightening reflexes and remaining on back (score 0), (Figure 2f) (this behavior not was not present in any other group). All the CB6F1 mice presented signs of being in Stage IV. In Figure 2, the different stages are registered as photos a–c (Stage 0), photos d–f (Stage I), photos g–h (Stages III) and photo i (Stage IV).
In Table 2, a summary of the scores of the behavioral parameters of the three mice groups is presented. These scores were taken when the parasitaemia percentage was at its highest point for each group of mice.
Parameters |
BALB/c |
C57BL/6 |
CB6F1 |
locomotor activity |
0 |
0 |
0 |
straightening reflex* |
3 |
3 |
0 |
pelvic elevation |
2 |
1 |
0 |
elevated tail |
0 |
0 |
0 |
exhaust reaction to finger pressure |
2 |
1 |
0 |
tremor |
0 |
0 |
1 |
piloerection |
1 |
1 |
0 |
deviation of head |
1 |
1 |
1 |
Parasitaemia(%) |
60±4,6% |
28±2,1% |
24±3,2% |
Mice with signs of MC |
0/10 |
8/10 |
10/10 |
Mice with brain blood barrier injure |
0/10 |
8/10 |
10/10 |
Table 2 Summary of scores of the parameters presented by the groups of mice used in the assay
Figure 3 shows the mice brains of the three strains used in the study, after perfusion with 2% Evans Blue. It can be observed that images 3b and 4b present an intense blue color, indicating Blood Brain Barrier injury.12
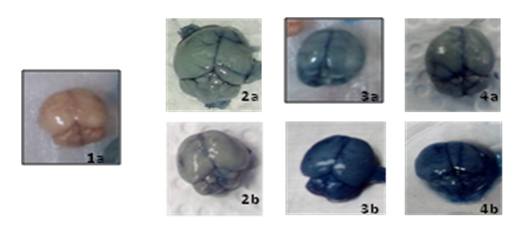
Figure 3 Photos of the mice brains of the three groups used in the assay, treaties with evan´s blue solution. 1a. Brain of healthy mice. 2a y 2b. Brains of BALB/c mice parasitized. 3a y 3b. Brains of C57BL/6 mice parasitized. 4a y 4b. Brains of CB6F1 mice parasitized.
The brain shown in 1a is representative of a healthy brain across all three strains. From 2 to 4 the pictures shown are representative of each group.
In Table 3 a summary of the ranges of parasitaemia and days correlated with Cerebral Malaria Stage is presented.
Neurological Stages |
Mice group |
Day ranges |
Ranges of |
Number of mice affected |
Injury of |
Stage I |
C57BL/6 |
0-3 |
0-11 |
10/10 |
* |
CB6F1 |
0-4 |
0-10 |
10/10 |
* |
|
BALB/c |
0-6 |
0–30 |
10/10 |
* |
|
>12% |
|||||
Stage II |
C57BL/6 |
3-5 |
11-20 |
6/10 |
* |
CB6F1 |
4-8 |
10-23 |
8/10 |
* |
|
BALB/c |
6-11 |
Started at 30% |
10/10 |
* |
|
Stage III |
C57BL/6 |
6-7 |
25 |
8/8* |
** |
CB6F1 |
8-9 |
22 |
10/10 |
*** |
|
Stage IV |
CB6F1 |
9 |
24 |
3/5* |
*** |
Table 3 Neurological Stages of the three groups of mice correlated with the time since initial infection, ranges of percentages of parasitaemia and number of mice affected
*Light **Moderate ***Considerable
Table 3. Neurological Stages of the three groups of mice correlated with the time since initial infection, ranges of percentages of parasitaemia and number of mice affected.
As evidenced in Table 3, the C57BL/6 and CB6F1 mice began presenting signs of CM in the range of 6 to 7 days post infection, with parasitaemias of 25% and 22%, respectively. After this period, the mice presented signs such as deviation of the head and exhaust reaction to finger pressure with scores between 1 and 0, and tremors with a score of 0. In this summary it can also be observed that BALB/c mice presented a higher parasitaemia (60%), but did not present neurological signs of cerebral malaria, although the brain showed some blue coloration (Figure 3: 2a, 2b). The results suggest that infection with Plasmodium berghei can affect many neurological functions in mice of the C57BL/6 and Cb6F1 strains.26
The results presented allow us to conclude that the SHIRPA protocol for behavioral parameters used in our study can evidence progressive functional and neurological deterioration caused by infection by the parasite. The C57BL/6 and CB6F1 mice both presented progressive manifestations of cerebral malaria, as determined by behavioral alterations which started with diminished locomotor activity and ended with head deviation and straightening reflex related to brain blood barrier injury. The CB6F1 mice group presented severe manifestations of Cerebral Malaria with a lower parasitaemia than the C57BL/6 mice. The study allowed us to establish the patterns of functional and neurological behavior that correlate with the percentages of parasitaemia, providing us with a model that manifests cerebral malaria with low percentages of parasitaemia, as occurs in humans. At the same time, the study allowed us to observe the differences in the behavior of strains that are susceptible and non-susceptible to Cerebral Malaria. The conclusion of this study is that BALB/c mice are non-susceptible to cerebral malaria, while C57BL/6 and CB6F1 mice are susceptible to cerebral malaria, making these last two strains suitable as models for malaria studies requiring these phenotypes.
The authors would like to thank everybody at the Center of Cellular and Molecular Biology of Diseases-INDICASAT AIP, for providing the facilities and means to carry out this study. Funds for this study were provided by SENACYT grants SNI 170-2016 and FID17-095.
The authors declare that there is no financial conflict of interest to the publication of these results.

©2018 Jesus, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.