MOJ
eISSN: 2573-2951


Research Article Volume 5 Issue 4
1Department of Poultry Diseases, Faculty of Veterinary Medicine, Cairo University, Egypt
2Department of Poultry Diseases, Veterinary, Research Division, National Research Center, Egypt
Correspondence: MM Amer, Department of Poultry Diseases, Faculty of Veterinary Medicine, Cairo University, P.O. Code 12211 Giza, Egypt
Received: July 25, 2018 | Published: August 21, 2018
Citation: Amer MM, Ahmed HM, Elbayoumi KM, et al. Pathogenicity of local identified NDV strain related to genotype VII to 28 day old commercial broiler chickens. MOJ Bioequiv Availab. 2018;2(4):247-250. DOI: 10.15406/mojbb.2018.05.00107
Twenty five broiler chicks were 28days old, chicken were infected by oculonasal route each with of 106 EID50 of local identified NDV local isolate (NDV/chicken/EG-QU/NRC/2015). Clinical signs begin at the 2nd dpi and at the 3rd dpi: marked depression with sleepy appearance, severe respiratory signs and marked decrease in feed intake, greenish lose dropping, beasty vent with greenish diarrhea, sudden high mortalities with death of 7birds in rate of 46.7%. At the 4th and 5th dpi same severe signs where greenish diarrhea was obvious with respiratory sounds were detected with death of 2birds (13.3%) in each. At 6th dpi death of 1bird (6.67%). The morbidity rate was 14/15(93.3%). while mortalities reach 80% in 4days. Post mortem examination of dead chickens in the infected group showed congested muscles, dehydrated, anorexic bird, hemorrhages on the tips of periventricular glands, liver congestion, greenish intestinal contents, greenish content of proventriculus and gizzard, severe necrosis and ulceration on cecal tonsils, and catarrhal tracheitis. NDV cloacal shedding was detected in cloacal swabs from infected chickens at 3(66.7%), 7(100%) and 10(66.7%) dpi by RT-PCR. Histopathological examination reveals the detection of showing necrosis of intestinal villi, hemorrhage and lymphocytic infiltration in intestinal sections. Cecal tonsils showed lymphocytic necrosis with areas of hemorrhage. Trachea showing Necrosis of lamina epithelialis, edema and hemorrhage in submucosa. Severe hemorrhage with severe lymphocytic depletion in spleen.
Conclusion: It can be concluded that the local identified NDV strain related to genotype VII is highly pathogenic to 28day old commercial broiler chickens.
Keywords: NDV genotype VII, pathogenicity, signs and lesions. virus shedding, broiler chicken
ND, newcastle disease; NDV, newcastle disease virus; VNDV, veloginic newcastle disease virus; SPF, specific pathogen free; RT-PCR, reverse transcription-polymerase chain reaction; ELD50, embero infective dose 50; HA, haemagglutination; HI, haemagllutination inhibition; ECE, embryonated chicken egg; dpi, days post infection
Newcastle disease is one of the most important avian viral diseases causing severe economic losses in poultry worldwide. Genotype VII associated with the most recent outbreaks in Asia, Europe, Africa, the Middle East and South America.1–3 Genotype VII had been reported in severeal countries in Asia such as China,4 Taiwan,5 Korea,6 Malaysia7 and also in' South America.8 Genotype VII (class II genotype VII) was firstly categorized into two sub genotypes: VIIa, which represents viruses that emerged in the 1990s in the Far East and spread to Europe and Asia; and VIIb, which represents viruses that emerged in the Far East and spread to South Africa. Later, the two sub genotypes of VII are classified into VIIc, d, and e, which represents isolates from China, Kazakhstan and South Africa; and VIIf, g, h, and i, which represent African isolates.9
Seal et al.,10 synthesized degenerate oligonucleotide primers used in a single-tube reverse transcription PCR to amplify nucleotide sequences from portions of the fusion protein and matrix protein genes of NDV genomic RNA and these techniques can be used reliably for ND epidemiology and for prediction of pathotypes of NDV isolates without traditional live-bird inoculations. Also, RT-PCR test was used to examine clinical samples from chickens experimentally infected with the NDV strain responsible for a recent epizootic and a positive correlation was obtained between the RT-PCR results and virus isolation for NDV from clinical samples.11 Kant et al.,12 used a semi-nested RT-PCR for the detection and subsequent differentiation of NDV isolates. Two oligonucleotide primers, representing the sequence at the cleavage site of the F protein of either virulent or non-virulent NDV strains, respectively, were used to differentiate NDV.
In Egypt, NDV continues one of the most predominant avian respiratory viruses since it was firstly isolated by Daubney et al.,13 till now causing severe economic losses in poultry industry.14,15 Since early 2011 NDV outbreaks occur in vaccinated and non-vaccinated poultry flocks in Egypt. F protein sequence analysis of NDV isolates and its phylogenic analysis revealed that these isolates belonged to genotype VII sub genotype d which related to China genotype.15,16–18 Four isolates of NDV genotype VII were isolated and identified by RT-PCR targeting the partial F-gene from severe outbreaks in commercial vaccinated poultry farms in Egypt.15 Now days, NDV outbreaks are still frequently occurring in vaccinated poultry flocks, despite the intensive vaccination programs.14,15,17–20 Therefore our study was carried out to investigate pathogenicity of local identified NDV strain related to genotype VII to 28day old commercial broiler chickens.
Fertile chicken egg
Specific pathogen free (SPF) eggs originated from Nile SPF (Koom Oshiem, Fayoum, Agriculture Research center- Ministry of Agriculture) were used for passage and titration of the isolated NDV isolates as well as preparation of HI antigen.
Experimental chicks
A total number 25 of day- old broiler chicks were kindly obtained from commercial hatchery were used for evaluation pathogenicity of isolate (NDV/Chicken/EG-QU/NRC/2015).15 Chicks were floor reared in previously cleaned and disinfected isolated rooms under hygienic conditions. Birds were provided with commercial broiler plated ration free from medication.
Newcastle disease viruses
Haemagglutination (HA) antigen
ND La Sota vaccinal strain were propagated in ECE and diluted to 4 HAU to be used as HA antigen in Haemagllutination inhibition (HI) testing of ND antibody.
NDV for pathogenicity study
Velogenic Newcastle disease virus (vNDV) local isolate NDV/Chicken/EG-QU/NRC/2015 (accession numbers MF418018) isolated and characterized by Hagar et al.15 The used strain is related to NDV Genotype VIId (Turkey-Israel-111-2011). It was titrated in SPF ECE as described by Grimes21 and EID50 was calculated according to Reed and Muench.22 The inoculation dose was 106 EID50/bird via oculonasal instillation.
Experimental design
Twenty five broiler chicks were reared in separated rooms; feed and water were provided ad-libitum. Blood samples for sera were collected from chickens at 1day of age and 28days (date of infection). At 28days old, chicken were infected by oculonasal route each chicken receive 0.2ml of 106 EID50 (NDV/chicken/EG-QU/NRC/2015). Infected birds were subjected to daily observation for 10days post infection (dpi) with recording of morbidity and mortality rates, clinical signs and pathological lesions. Viral antigen was detected in tissues and cloacal swaps for viral shedding by RT-PCR on samples from 3birds/group at 3, 7 and 10 dpi. Blood samples were taken to determine level of HI antibodies against NDV. Histopathological lesions were detected in tissue samples collected from freshly dead birds at the 5th dpi. The obtained results are shown in Table 1, Table 2, Figure 1 and Figure 2.
No of birds |
Distribution of morality/days post infection |
|
|
Total |
% |
||||
|
1st |
2nd |
3rd |
4th |
5th |
6th |
7th-10th |
|
|
15 |
---- |
---- |
7 |
2 |
2 |
1 |
---- |
12 |
80 |
% |
0 |
0 |
46.7 |
13.3 |
13.3 |
6.67 |
0 |
|
|
Table 1 Distribution and total mortalities of infected chickens
Day post infection |
RT-PCR result |
||
|
No of +ve |
No of -ve |
% of +ve |
3 |
2 |
1 |
66.7 |
7 |
3 |
0 |
100 |
10 |
2 |
1 |
66.7 |
Table 2 RT-PCR results of cloacal viral shedding (n=3)
Conventional RT-PCR for detection of NDV cloacal shedding
The genomic viral RNA was directly extracted from cloacal swabs by using QIAamp viral RNA extraction Kits according to the manufacture’s protocol. One step RT-PCR was carried using QIAGEN® One Step RT-PCR kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. RT-PCR was used for the detection of vNDV shedding as previously described by Seal et al.,10 Wise et al.,11 and Kant et al.12
Histopathological examination
Affected organs including the trachea, spleen, cecal tonsils and intestine from the positively infected farms were collected and fixed on 10% formol saline. The specimens were routinely processed in paraffin embedding method, sectioned and stained with Haematoxylin and Eosin (H&E) for light microscopic examination according to Bancroft and Gamble.23
Avian paramyxovirus type 1 or ND viruses are frequently recovered from wild birds and such isolates are most frequently of low virulence. Velogenic NDV are usually recovered from poultry and only occasionally from wild birds. VIId is the predominant pathogen involved currently in ND outbreaks in poultry worldwide.24–27 Recent findings suggest that the virulent virus may emerge in poultry as a result of mutations in viruses of low virulence.24 Mean HI titer was 8.5±1.19 and 1±1.06 at 1 and 28days of age 18.
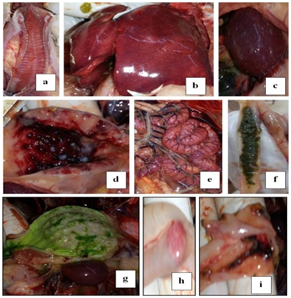
Clinical signs begin at the 2nd dpi with slight depression and decrease in feed intake with no mortality. In 3rd dpi: marked depression with sleepy appearance "Figure 1A" and marked decrease in feed intake, greenish lose dropping "Figure 1B", Basty vent with greenish diarrhea "Figure 1C", sudden high mortalities "Figure 1D".18,25,28
Mortalities " Table 1" start at the 3rd dpi with death of 7birds in rate of 46.7%.18,26,28 At the 4th dpi same severe signs were detected with death of 2birds (13.3%). In 5th dpi: greenish diarrhea was obvious with respiratory signs with death of 2birds (13.3%). At 6th dpi death of 1bird (6.67%). From 7th to 10th dpi paresis in 2 of remaining live chickens and one bird was healthy. Therefore the morbidity rate was 14/15 (93.3%). while mortalities reach 80% in 5 dpi.18,26,28 Endemic ND can cause mortality of up to 60% in village chickens.25

Post mortem examination of dead infected chickens "Figure 2" showed dehydrated, anorexic bird, Catarrhal tracheitis with hemorrhagic mucosa "Figure 2A", Congested enlarged liver with sub capsular hemorrhage "Figure 2B", Enlarged mottled spleen "Figure 2C" hemorrhages on the tips of periventricular glands "Figure 2D", Congested serosal surface of intestine "Figure 2E", liver and muscle congestion, greenish intestinal contents "Figure 2F" , greenish content of proventriculus and gizzard "Figure 2G", Hemorrhagic cecal tonsils serosal view "Figure 2H" Necrosis and ulceration in mucosal of cecal tonsils "Figure 2I". These signs and gross lesions agree with those observed by Awad et al.,14 El-Behairy et al.,18 Moussa,28 Susta et al.,29 and Abd El Aziz et al.30
NDV cloacal shedding was detected at 3, 7 and 10 dpi by examining cloacal swabs "Table 2" from infected chickens for detection of viral shedding by RT-PCR where percentage of postive results was 66.7%, 100% and 66.7%; respectively.3,28,31,32
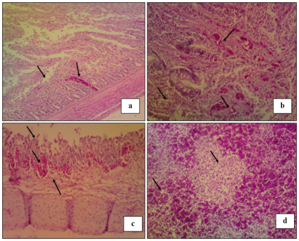
Histopathological examination "Figure 3" reveals the detection of showing necrosis of intestinal villi, hemorrhage and lymphocytic infiltration in intestinal sections "Figure 3A". Cecal tonsils showing lymphocytic necrosis with areas of hemorrhage "Figure 3B".Trachea showing necrosis of lamina epithelialis, edema and hemorrhage in sub mucosal layer "Figure 3C". While, Spleen showing severe hemorrhage with severe lymphocytic depletion "Figure 3D".18,28
It can be concluded that the local identified NDV strain related to genotype VII is highly pathogenic to 28 day old commercial broiler chickens.
This study was carried out according to Guiding principles for the care and use of animals for scientific purposes and in accordance with local laws and regulations.
Authors are grateful to thank Dr. Mohammed Bosila, Department of Poultry. Diseases, Vet. Res. Division, National Research centre for his help in histopathology.
The author declares that there is no conflict of interest involved in this study.

©2018 Amer, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.