MOJ
eISSN: 2573-2951


Mini Review Volume 5 Issue 3
Chemistry Department, DePaul University, USA
Correspondence: Youya Gao, Chemistry Department, DePaul University, Chicago, 1110 West Belden Avenue, Chicago, IL, 60614, USA, Tel 847-220-1493
Received: March 23, 2018 | Published: May 2, 2018
Citation: Gao Y. Introduction and synthesis of polymeric prodrugs. MOJ Bioequiv Availab. 2018;5(3):128-131. DOI: 10.15406/mojbb.2018.05.00092
The conventional drug delivery system with small molecular weight usually exhibits shorter half-life, non-specific target in the blood stream and higher overall clearance rate. Thus, higher doses are often required for therapy purposes and may cause terrible sides effects like toxicities on the body. Due to the organ toxicities caused by these unfavorable physical and chemical properties, new drug delivery systems such as polymeric with higher bioavailability and less side effects are in highly demand. In this mini review, we aid at bringing up an overview of polymer prodrugs with their advantages and shortages.
Keywords: new drug delivery system, polymeric prodrug, bioavailability, site effect, ringsdorf’s model, site-specific, organ toxicity
VEGF, vascular endothelial growth factor; RGD, arginine-glycine-aspartic acid; PEG, poly ethylene glycol; AUC, area under curve; DCC, dicyclohexyl carbodiimide; DVS, divinyl sulphone; 6-ACA, 6-amino caproic acid
Conventional drug delivery systems (tablets, capsules, pills, creams, ointments, liquids, aerosols) with small molecular weight usually involve non-specific administrations. High doses are required because of their unfavorable physical and chemical properties such as: lower bioavailability, shorter biological half-life, and drug resistance. These properties may cause terrible side effects on the tissues like toxicity.1,2 To overcome these problems, scientists have focused on polymers, especially biomedical polymers which are used for developing specific targeting drug delivery systems during the past two decades.3 Recently, two possible ways are found out: the use of new therapeutic substances or new delivery systems. Apparently the second approach is preferred because the discovery and development of new active molecules are more difficult and expensive. As a result, significant improvements in the new delivery systems have been shown with pharmacokinetic and pharmacodynamic profiles.
These new approaches are based on the preparation of favorable genetic variants, or tailor-made formulations, such as liposomal preparations,4 controlled release systems,5 and covalent modifications of the drug by low molecular weight reagents or by polymer conjugations.6 The new macromolecular delivery systems using polymers as prodrugs are come up to reduce site effects and at the same time to increase the efficiency of many respective drugs that popular in the clinical use.7 This mini review provides a perspective on the design principles and properties of polymeric prodrugs as further applications on diseases.
A conjugation of a drug with a polymer forms so-called "polymeric prodrug". A prodrug is a form of a drug that remains inactive during its delivery to the site of action and then is activated by the specific conditions at the targeted site. In other words, a prodrug is an inactive precursor of a drug. The concept of the prodrug was first suggested by Albert and Harper.8–10 Such polymer conjugation is a fast-growing technique that already produced several molecules available in the market, as shown in Table 1.
S. no. |
Conjugate |
Indication |
Marketing year |
Company |
1 |
PEG– adenosine deaminase (Adagen) |
SCID syndrome |
1990 |
Enzon |
2 |
PEG– asparaginase (Oncaspar) |
Acute lymphoblastic leukaemia |
1994 |
Enzon |
3 |
SMANCS (Zinostatin, Stimalamer) |
Hepatocellular carcinoma |
1993 |
Yamanouchi Pharmaceutical |
4 |
Linear PEG– interferon α2b (PEG–Intron) |
Hepatitis C, clinical evaluation on cancer, multiple sclerosis and HIV/AIDS |
2000 |
Schering Plough/Enzon |
5 |
Branched PEG– interferon a2a (Pegasys) |
Hepatitis C |
2002 |
Roche/Nektar |
6 |
PEG–growth hormone receptor antagonist |
Acromegaly |
2002 |
Pfizer (Pharmacia) |
7 |
PEG–G-CSF (Pegfilgrastim, Neulasta) |
Prevention of neutropenia associated with cancer chemotherapy |
2002 |
Amgen |
8 |
Branched PEG–anti- VEGF aptamer |
Age-related macular degeneration |
2004 |
(OSI Pharmaceutical)/Pfizer |
9 |
PEG–anti-TNF Fab |
Rheumatoid arthritis and Crohn’s disease |
2008 |
UCB (formerly Celltech) |
Table 1 Polymer-drug conjugates available in market
Polymer materials are designed to be capable of delivering active substances to the target diseased tissues and cells. Prof. H. Ringsdorf developed a rational model (Figure 1) of polymeric prodrug for the first time in 1975. And Prof. Ringsdorf was the first scientist to recognize the huge potential of polymeric prodrugs, allowing polymer chemists and biologists would work together in the field.11
The proposed model consists mainly of five components: the polymeric backbone, the drug, the spacer, the targeting group and the soluble agent. The backbone could be either an inert or a biodegradable polymer. The role of spacer is to control the site and the rate of releasing the active drug from the conjugate by hydrolytic or enzymatic cleavage. The drug must be covalently bonded and must remain attached to the polymer until this macromolecule reaches the target site. So, the drug chosen must be potent and intact until it reaches the target. Besides, it should have a functional group to bind with the polymer backbone directly. The targeting moiety or homing device guides the entire polymer-drug conjugate to the targeted tissue. Now, in general, there are different kinds of the therapeutic agents could be incorporated into polymer chain, end-capped or formed a pendant group of the macromolecular chain.6,12 (Figure 2).
Prolongation of drug action
The duration of the drug is determined by its plasma concentration which is usually measured as area under curve (AUC). The drugs which have slow renal elimination and are metabolically inactive have prolonged duration of action. This conjugation results in a slower renal excretion, longer blood circulation and an endocytotic cell uptake.10
Controlled drug release
The polymeric prodrug is stable in the circulation but also able to release the drug both intra-cellularly and intra-tumorally for a therapeutic purpose. This controlled release from polymeric prodrug can only be achieved by the proper selection of linkages between drug and polymeric carrier.5
Immuno-protection
Cancer treatment by polymeric prodrug can remarkably protect the patient’s immune system owing to a mechanism known as Fas-Fas ligand interaction. Fas and Fas ligand (Fas L) are present on cancers as well as immune cells. Interaction between Fas and Fas ligand (Fas L) triggers a cascade of signals, that eventually results in programmed cell death. It has been reported that the treatment with free anti-tumor drugs promotes induction of Fas ligands on cancer cells whereas their macromolecular derivatives do not increase Fas L. This is an important outcome that might indicate that polymeric prodrugs can protect the patient’s immune system.13
Active targeting by polymeric prodrug14
Monoclonal antibodies
The monoclonal antibodies can be used as targeting group for coupling with the drug to increase the specific targeting of the prodrug on the tumor cells. These antibodies bind very specifically to tumor cells and this approach has been successfully used in cancer therapy. For example:
Lectins
The sugar specific receptors present on the plasma membrane are called lectins and they have been characterized mainly on hepatocytes. Galactose specifically targets these lectins and this targeting seems to be an attractive approach for target specific drug delivery especially for treatment of liver diseases such as hepatitis, parasitic infections and liver metastasis. Drug delivery to macrophages (e.g. Kupffer cells) can be employed for targeted treatment of various malfunctions such as leishmaniasis, Gaucher’s syndrome etc.
Angiogenic vessels of tumor cells
The endothelial cells in angiogenic vessels of tumors show increased expression of cell surface proteins. These proteins include receptors for vascular endothelial growth factor (VEGF) and integrin receptors. The peptides which specifically bind to these receptors can be used as targeting moiety for drug delivery such as RGD (arginine-glycine-aspartic acid) containing peptides that specifically bind with integrin receptors. The conjugation of RGD peptides and poly (ethylene glycol) (PEG) showed increased efficacy of drug against breast cancer.
Requirements for selecting polymers as drug carriers:
Classification of polymers used for bioconjugation
Coupling methods for the synthesis of polymeric prodrugs
Coupling reactions involves conjugation of drugs with polymers. Coupling agents mediate the conjugation of the two molecules by forming a bond with no additional spacer atom. The most commonly used coupling agents are dicyclohexyl carbodiimide (DCC), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, EDCI) and N-hydroxysuccinimide esters. Drugs or other effective agents are chemically conjugated to polymers through ester, amide or disulphide bonds. The bonding should be relatively stable to prevent drug release during its transportation before reaching the targeted site.
Recent advancements in coupling methods involve the use of homobifunctional amine or heterobifunctional coupling reagents. A few electrophilic groups such as epoxides, vinylsulphones, and aziridines can react with amines and other nucleophiles. Homobifunctional compounds such as N, N’- ethyleneiminoyl- 1-6-diaminohexane, bis-aziridin, divinyl sulphone (DVS), nitrogen mustard and bis-sulphonyl chloride can form protein-protein linkages while heterobifunctional reagents are useful in coupling amines with other functional groups. Reactive groups in protein-carboxyl functions offer alternating thiol reactions as a site for hetero bifunctional coupling with amines.
Applications
Coupling of polymers containing hydroxyl groups to alcohols and amines: Polymers containing -OH groups (e.g. PEG) can be modified to Carboxylic acid derivative by treating with acid anhydrides. PEG or mPEG can be acetylated with anhydrides (e.g. succinic anhydride) to form an ester terminating to free carboxylate groups. The resulting succinylated derivative containing free -COOH group can be further used for conjugation with drugs or proteins (Figure 3). The succinyl group incorporated in the polymer may act as a spacer between the polymer and the drug which may control the site and the rate of releasing of the active drug from the conjugate by hydrolytic or enzymatic cleavage.

Figure 3 Conversion of PEG to Succinylated PEG containing free -COOH group and coupling of succinylated PEG to amine and alcohol using a carbodiimide as coupling agent to form
A: amide bond; B: ester bond conjugate.
Coupling of drug and polymer through a spacer: Spacers may be incorporated during bioconjugation to decrease the crowding effect and steric hindrance and thus to control the site and the rate of releasing the active drug from the conjugate. Spacers can enhance the affinity of ligand-protein binding. Amino acids such as glycine, alanine, and small peptides are widely used as spacers due to their chemical versatility for covalent conjugation and biodegradability. The α-amino acids in peptides and proteins (proline excluded) consist of a carboxylic acid (-COOH) and an amino (-NH2) functional group attached to the same tetrahedral carbon atom which extends the diversity for conjugation with hydroxyl, carboxyl or amino groups of polymer or biomolecule. Moreover, amino acid based- spacers are short-chained, reactive, and biocompatible and may release the active agent from the conjugate. Di-functional amino acids such as 6-amino caproic acid (6-ACA) or 4 amino butyric acids (4 ABA) have been used as spacer arms between the polymers and the ligands for applications in biotechnology. A polymeric prodrug of alkylating agent mitomycin C (MMC) with poly[N5-(2-hydroxyethyl)-L-glutamine] (PHEG) using oligopeptide spacers is designed predominantly for enzymatic degradation which released MMC with a dependent manner.15–17 (Figure 4).
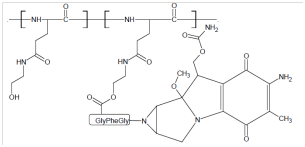
Figure 4 A polymeric prodrug of alkylating agent mitomycin C (MMC) with poly[N5-(2-hydroxyethyl)-L-glutamine] (PHEG) using oligopeptide spacers.
Limitations and problems in the synthesis
It is now remarkable that the bioactive components of a macromolecular drug can be delivered more efficiently by converted into prodrug forms than original forms. A number of publications and patents have been indicated the crucial role of this technique in drug delivery and therapeutics. Modification of biological macromolecules, anticancer drugs, peptides, and proteins in the form of polymeric prodrugs play extremely important roles in therapeutics. While, the potential of polymeric prodrugs acting as site-specific delivery systems still need to be deeply explored for more specific details. It is believed that the technique of polymeric prodrug conjugation is a fascinating approach for efficient drug delivery and appears to have a bright future in healthcare.
None.
The authors declare there is no conflict of interest.

©2018 Gao. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.