MOJ
eISSN: 2573-2951


Transdermal drug delivery system (TDDS) is a dosage form designed to deliver drug through skin by topical route of administration to the systemic circulation or for local treatment. As a new type of dosage form, TDDS has a number of special merits, but inevitably at the same time it possess some distinct disadvantages, such as low permeability and the drug mostly enter the systemic vascular. Microsponges are a kind of micro-sized and polyporous particles which can entrap effective constituents, and are biologically safe. And Microsponges also exhibit distinctive advantage at the aspect of drug release. The TDDS can enhance drug stability, decrease side effects, improve bioavailability and so forth, which has been used mostly through topical and oral administration, and applied broadly in medical and cosmetic field. This review presents application of microsponges in TDDS including its preparation, properties and drug-released studies in the recent advancements.
Keywords: bioavailability, microsponge, transdermal drug delivery system, preparation, characterization, drug-released studies
TDDS, transdermal drug delivery system; FDA, food and drug administration; EC, ethyl cellulose; BPO, benzoyl peroxide; SEM, scanning electron microscopy; AUC, area under the curve; EE, entrapment efficiency; DSC, differential scanning calorimetry; MED, minimal erythemal dose; SPF, sun protection factor
Compared with drug dosage forms for oral and parenteral administration, Transdermal drug delivery system (TDDS) can minimize and avoid some restrictions relating to dosage forms effectively. Due to the conventional delivery systems suffering from certain restrictions like Peak and Valley phenomenon i.e., they show fluctuations in plasma drug concentration and cannot render sustained effect.1 However, the TDDS can meet the requirement to keep relatively stable plasma drug concentration and prolong delivery of drug, in a steady-state profile and minimizes the peak-associated side effects, which thus ensures the level of the drug is effective for treatment on the minimal therapeutic concentration. In general, Transdermal drug delivery system, as a controlled drug delivery system, is exceedingly humanized, commodious and prolong the period of time of release of drug, if need arises (e.g. systemic toxicity) with less pain sensation while administrating drug candidates.2 Additionally, TDDS permits the permeation of drugs across the skin and into the systemic circulation which avoids the hepatic first-pass effect observed in the course of oral administration and inconvenience of frequent parenteral administration.3
As a new drug carrier of TDDS, Microsponges are mostly used for topical4-10 and oral administration.11-15 For other topical delivery systems, the traditional delivery systems are required of frequent dosing to keep relatively stable local concentrations of effective constituent for therapeutic effectiveness because of low effectiveness. Thus to avoid this defect it is indispensable to design a new drug delivery system to prolong the residual time of drug in the epidermis or the dermis, while reduce its penetration into the body.7
For this reason, as a porous polymeric microsphere, Microsponges can fulfill such aims uniquely.4 Microsponges are porous, polymeric microspheres (Figure 1) which are invariably used for topical delivery system;4-5,7-10 its inside are full of lacunas and its particle size is 5~300μm in diameter.
As a drug carrier for skin-target delivery, microsponges have several advantages:
At the beginning, microsponges were used in oral drug delivery system. For example, as early as1989, Kawashima17 had prepared the ibuprofen microsponges by using quasi-emulsion solvent diffusion method, and since then, this kind of method for preparation of microsponge was commonly used.18-21 And subsequently, the advantages of microsponges were found and revealed by researches gradually.
In the early time, the microsponges used in Transdermal drug delivery system was benzyl peroxide microsponges in 1991.5,22 And later, the Retin-A microsponges (0.1% or 0.04% tretinoin) and Carnac microsponges (0.5% 5-flurouracil) for acne and actinic keratoses respectively were approved by Food and Drug Administration (FDA). Afterward, microsponges for topical skin-target drug delivery are widely developed and utilized in topical drug delivery system, such as mupirocin microsponge, hydroxyzine hydrochloride microsponge, paeonol microsponge and so on.
Usually, microsponges were prepared mainly by two methods. One is a quasi-emulsion solvent-diffusion method,7,8,23 and another is free-radical suspension polymerization. But, irradiation, high temperature, or catalysis to activate the monocase is indispensable reaction condition16 when microsponges were prepared by free-radical suspension polymerization, and the manufacturing processes are complex and reproducibility is poor. Compared with this way, the quasi-emulsion solvent-diffusion method is not only easily performed in the laboratory, but also has the potential to prepare microsponges readily in the factory.7,24
In the process of preparation, in order to optimize the preparation process of microsponges7-9,25,26 and improve release of drug,27-28 the factors including drug/polymer ratio, disperse phase solvent amount, stirring time, speed and stirrer type on the preparation of microsponges always need to be investigated. Afterwards, the thermal behavior, surface morphology, particle size and pore structure of microsponges should be examined. Eventually, for the purpose of evaluating the effect of microsponges, we always carry out in vitro 24 and in vivo drug release studies.7,8,10
In 2006, Jelvehgari et al.24 produced ethyl cellulose (EC) micro particles containing benzoyl peroxide (BPO). BPO micro particles were prepared by an emulsion solvent diffusion method. In the process of preparation (Figure 2), the researcher investigated such factors: drug /polymer ratio, stirring rate, volume of disperse phase, and found that those factors influenced the particle size, drug content and drug release behavior of microsponges.29-31
In addition, the release dynamic characteristic of BPO from microsponges was discussed. The author conducted the release studies using Franz diffusion cells (ERWEKA, Germany), and the result showed that the kinetics of the release of BPO from microsponges corresponds to zero-order dynamic model. Afterwards, samples were withdrawn at different time points and was analyzed using HPLC. Each test was carried out in triplicate and the mean of three observations was reported. The data of drug release indicated that the rate of initial hour was higher than that of the second hour. Then, within the next 7h, the flux remained constant, which showed the entrapped drug released from microsponges.
Later, in 2011, Hydroxyzine Hydrochloride10 as a drug model, which was used to produce a novel drug-loaded dosage form-microsponges that could control the release of drug into the skin. The method of preparation of Hydroxyzine HCl microsponges was a little different from the method of preparation of BPO microsponges.
When microsponges were prepared by the said method (Figure 3), the following factors could be used to optimize the preparation of drug microsponge:
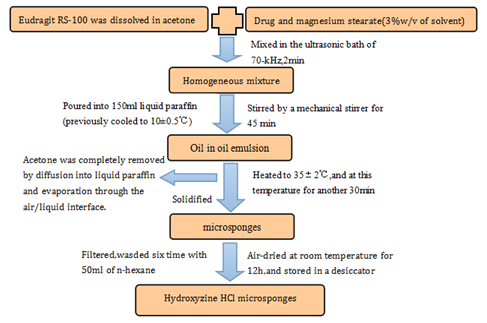
The researcher evaluates preparation of Hydroxyzine HCl microsponges by the entrapment efficiency calculated through the following formula: (Practical drug loading/Theoretical drug loading) × 100.
Unlike previous mentioned in vitro release studies, this article used cellulose nitrate membrane instead of animal skin to perform the study of drug release. In addition, the research assayed the drug content of microsponges with spectrophotometer at λmax=231 nm32,33 rather than HPLC.
The procedure of drug diffusion was divided into two parts. Firstly, the aqueous solution was diffused to the matrix and drug was dissolved; secondly, the drug from the pores spread to the dissolution medium. Thus, the release curve (Figure 4) of Hydroxyzine HCl from the microsponge indicated that the process has two obvious phases. In the first three hours, the rate of release of drug increases gradually and approaches closely to the highest rate, then followed by a placid release phase. In the in vivo studies, the author detected pharmacodynamic activity of antihistamines by percutaneous injection of histamine in skin of subjects, and the skin was treated by this microsponge, which was able to restrain wheal and flare response induced by histamine. There are two groups (n=4/group) used in this study, Group I (dealt with blank microsponges) and Group II (dealt with medicated products)
Compared with the innovative wheal areas, the rabbit of Group I cannot be cured, but another group can be cured after 4h (Figure 5). It showed that wheal and flare can be cured by the medicated microsponge. In 2013, the group of Liu et al.8 firstly put forward a new skin-target drug delivery system containing Paeonol which was extracted from the root of Paeonia suffruticosa, a kind of traditional Chinese medicine. The preparation of Paeonol microsponges is the same as flurbiprofen microsponges,15 using the quasi-emulsion solvent-diffusion method (Figure 6).
In this paper, the researcher evaluated the feature of the paeonol microsponges from the followed factors: scanning electron microscopy (SEM), yield, entrapment efficiency, and particle size, and then prepared paeonol microsponges into a cream to analyze and research on release of paeonol in vitro and in vivo.
The Franz diffusion cells were used to carry out the release studies in vitro, and the content of drug samples withdrawn at different time points were analyzed by HPLC.
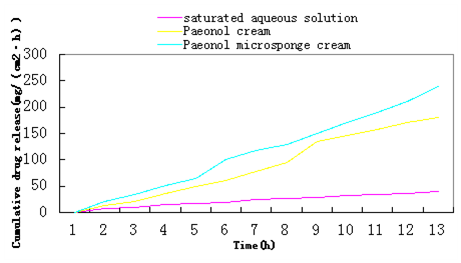
In contrast, the flux (Jss) of Paeonol microsponge cream was (21.17±0.284)mg/cm2/h, higher than Paeonol cream (17.29±0.312)mg/cm2/h and saturated aqueous solution of paeonol (3.46±0.091)mg/cm2/h (Figure 7). The zero order (R2=0.997) of Paeonol microsponge cream was the highest among three different formulations, and the zero order was higher than first order and Higuchi. Thus, the result proved that the kinetics of in vitro release curve of paeonol from microsponge cream satisfied the zero order kinetics.
In ex vivo drug-residues studies, the amount of paeonol from paeonol microsponge cream stored in the skin was almost one and a half times than the paeonol cream at 24h (1.3627±0.0699 mg/cm2 vs. 0.9988±0.0801 mg/cm2). The result showed that microsponge delayed the release of drug into the skin.
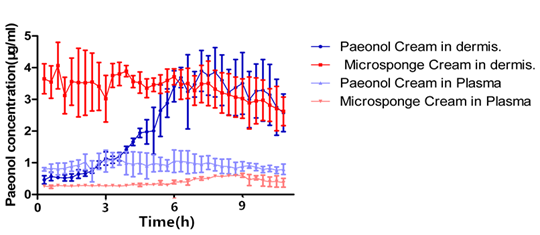
In this article, the intra-dermal micro dialysis was used in the in vivo release studies among different formulations of paeonol.
The mean t1/2 for the paeonol microsponge cream was 935.1 min, while was 548.6 min for the paeonol cream, indicating that the microsponge form has a good distribution into the skin. In addition, the drug content of paeonol microsponge cream was extremely steady compared with paeonol cream (Figure 8), helping the drug to be released in specific area. It is useful for treating dermatosis because the drug was reserved as much as possible in the treated areas. The area under the curve (AUC) measured by micro dialysis showed that the paeonol microsponge could deliver a mass of paeonol to the skin, and thus improved its bioavailability. Microsponges may be a promising skin-target drug delivery system for treating dermatosis.
Oxybenzone is a broad-spectrum sun-screening agent generally used in the formulation of cream, gel and lotion. In 2014, Pawar and coworker9 formulated Oxybenzone loaded microsponge gel to improve the efficacy of sun protection, and reduce toxicity. The preparation of Oxybenzone microsponge was similar to BPO microsponge, and the method was the quasi-emulsion solvent-diffusion method (Figure 9).
In order to evaluate the optimized formulation, researcher investigated the following variables: physiochemical characterization, yield, particle size, percent entrapment efficiency (%EE), drug release, surface topography, and differential scanning calorimetry (DSC). Next, the study also examined rheological characterization, skin irritation, minimal erythemal dose (MED), and sun protection factor (SPF) determination to evaluate the prepared gel.
By USP dissolution test apparatus, researcher detected the rate and extent of drug release from Oxybenzone loaded microsponge. The percentage of drug released at different time intervals was calculated and plotted against time.
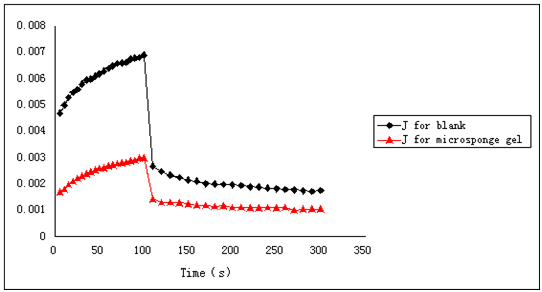
The optimization of Oxybenzone loaded microsponge gel showed that the preparation was successful and enhanced sun-screening efficiency. Additionally, the creep recovery of blank microsponge was 65.11%, while drug loaded microsponge gel was 74.05% (Figure 10). As we known, the high creep recovery was helpful for promising gelling with higher elasticity. Meanwhile, SPF was increased and topical retention of drug was also enhanced in drug loaded microsponge gel, indicating the microsponge gel is remarkable in the in vitro and ex vivo evaluation study.
Microsponges are increasingly drawing attention from researchers all over the world due to the advantages of extending the residual time of drug in the epidermis or the dermis, while reducing its penetration into the body. In addition, microsponges can reduce skin irritation and other side effects and frequency of drug application, and improve efficacy and patient compliance. We believe that microsponge-based emulgel formulations would play a crucial role in the treatment of skin disease and at the aspect of development and progress of traditional Chinese medicine, and it will be a potential area to attract more and more pharmaceutical researchers to engage in TDDS study in the future.
We sincerely thank the Natural Science Foundation of China (81373975), GuangDong College Chinese Cosmetics Engineering Centre Construction (GCZX-A1007), Guangdong Natural Science Foundation (2014A030310342) and the Youth Training Program of Southern Medical University (PY2013N009) for financial support.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.