MOJ
eISSN: 2471-139X


Research Article Volume 2 Issue 6
1Department of Biomedical Sciences, Ohio University, Heritage College of Osteopathic Medicine, USA
2Department of Specialty Medicine, Ohio University, Heritage College of Osteopathic Medicine, USA
3The Diabetes Institute at Ohio University, USA
Correspondence: Kelly McCall, Department of Specialty Medicine, Ohio University, Heritage College of Osteopathic Medicine, USA, The Diabetes Institute at Ohio University, USA
Received: June 30, 2016 | Published: August 2, 2016
Citation: Wood A, Claybaugh T, Slyvka. The efficacy of niacin-bound chromium to slow the progression of diabetic nephropathy in type ii diabetic rats. MOJ Anat Physiol. 2016;2(5):156–160. DOI: 10.15406/mojap.2016.02.00064
Animal and clinical studies suggest niacin-bound chromium (NBC) added to the diet improves glucose-insulin sensitivity, the ability to ameliorate inflammation, hypertension, and proteinuria. In addition to its lipid-lowering actions, NBC possesses potent antioxidant and anti-inflammatory properties. Rat studies demonstrated that T2DM decreases both the production and bioavailability of nitric oxide (NO), and we hypothesized that NBC supplementation will preserve NO production via increased endothelial nitric oxide synthase (eNOS) and decreased neuronal nitric oxide synthase (nNOS). NBC supplementation decreased levels of plasma creatinine in the treated diabetic rats compared to the untreated diabetic rats, and therefore preserved glomerular filtration rate (GFR). We provide evidence that this effect may also be due to increased levels of eNOS, which leads to renal microvascular vasodilation. Supplementation of NBC also lowered nNOS levels, which is a significant finding when considering the role of nNOS in T2DM. Marked improvements in fasting glucose and lower body weights were observed in the treated diabetic rats compared to the untreated diabetic rats. Therefore, these results demonstrate that NBC supplementation slows the progression of diabetic nephropathy (DN) in type 2 diabetic rats.
Keywords: insulin resistance, diabetes mellitus, diabetic nephropathy, hyperfiltration, nitric oxide, niacin-bound chromium, efferent arterioles, tubuloglomerular, vasodilation, lipid metabolism, ameliorate hypertension
NBC, niacin-bound chromium; NO, nitric oxide; GFR, glomerular filtration rate; DN, diabetic nephropathy; CGMP, cyclic guanosine monophosphate
Insulin resistance-related disorders such as type 2 diabetes mellitus (T2DM) and obesity have reached epidemic proportions in the recentyears.1 Diabetic nephropathy (DN) is the number one cause of end-stage renal failure.2 The pathogenesis of DN perhaps occurs in two major stages: First, there is a vasodilation in the pre- and post-glomerular arterioles, which leads to higher intra-glomerular blood flow and pressure. This causes an early hyper filtration in the nephrons and eventually damages the glomerular membrane and allows for proteins, glucose and other molecules to be filtered by the glomerulus and then excreted in the urine. Secondly, the final irreversible stage is a vasoconstriction of the glomerular arterioles, which results in low blood flow and glomerular filtration rates (GFRs).3 The nitric oxide (NO) system has been shown to be altered in diabetes and in diabetic nephropathy.4 NO is an important bioactive signaling molecule that mediates a variety of normal physiological functions, which, if altered, could contribute to the genesis of many pathological conditions, including diabetes.5 We have previously reported alterations in the renal NO system in type 2 diabetic rats.6 Niacin-bound chromium (NBC), at adequate dosing, increases insulin sensitivity, enhances NO activity, and appears to be non-toxic.7 Endothelial nitric oxide synthase (eNOS) results in NO release from the endothelium of blood vessels and causes vasodilation via cyclic guanosine monophosphate (cGMP).8 Neuronal nitric oxide synthase (nNOS) is constitutively expressed in the kidney in the macula densa and endothelial cells of the efferent arterioles. It has also been demonstrated that NO derived from nNOS plays an important role in counteracting modulation of tubuloglomerular feedback (TGF)-mediated afferent arteriolar constriction.9,10 Compared with normal rats, diabetic rats demonstrated enhanced renal hemodynamic responses to nNOS inhibition, suggesting increased basal activity of nNOS in the diabetic kidney.11 Therefore, higher levels of renal eNOS compared to nNOS would be beneficial in the late stages of diabetic nephropathy to maintain renal blood flow via vasodilation. In the later stages of diabetic nephropathy, the GFR decreases. This is due to significant renal vasoconstriction. The continued availability of NO would maintain GFR and renal blood flow. It has been presumed that in the kidney, eNOS predominates in the delivery of NO. Also, in recent studies, renal hemodynamics appears to be more sensitive to nNOS inhibition in diabetic rats.11 In the present study, we hypothesized that NBC supplementation would increase NO bioavailability since it is upregulated by eNOS and by the inhibition of nNOS in the later stages of diabetic nephropathy. There is no clinically defined state of chromium deficiency, but diabetes has been shown to develop because of low chromium levels in experimental animals and in humans. These results suggest that there may be a more general relationship between chromium levels and glucose and/or lipid metabolism.12 Niacin (nicotinic acid) is a water soluble vitamin of the B group. It is an essential nutrient required for the proper metabolism of carbohydrates, lipids and proteins.13 In addition to the favorable effects on lipoproteins, several findings support the idea that niacin therapy may ameliorate inflammation. Niacin appears to attenuate the overexpression of NOS in a rat model of silica-induced lung inflammation.14 NBC can ameliorate hypertension, dyslipidemias and diabetes mellitus, and may be useful in weight management.15 Therefore, the hypothesis of this study was that a NBC diet would preserve renal function and slow the progression of diabetic nephropathy in a rat model of obesity-related T2DM. To test this hypothesis, we utilized the Zucker rat model of T2DM,16‒22 and evaluated the effects of NBC supplementation on several components in T2DM: renal eNOS and nNOS, plasma creatinine, fasting blood glucose, and body weight. We report the novel findings that NBC supplementation preserved renal function, slowed the progression of diabetic nephropathy, and lowered nNOS levels in male Zucker rats, suggesting that NBC supplementation improves renal function by lowering nNOS levels.
Type 2 Diabetic rat model and groups
All animal work was conducted with approval from the Ohio University Institutional Animal Care and Use Committee. Our experiment included two genetically different groups of rats. One group was the obese Zucker rat (fa/fa). The second group was the lean Zucker rat (Fa/Fa). The obese Zucker rats are genetically leptin receptor deficient. This causes the animals to become hyperphagic, obese, and develop metabolic characteristics similar to that of T2DM.22 The male obese Zucker rats (n=16) and lean Zucker rats (n = 8) were obtained from Harlan Laboratories (Indianapolis, IN) at 4weeks of age. Animals were housed in a climate-controlled vivarium with a 12:12 hour light-dark cycle and fed a standard laboratory diet libitum (Purina Mills, Brentwood MO) and water ad libitum for 4days while the animals acclimate to laboratory conditions. After being acclimated to laboratory conditions for 4days, they were randomly divided into three experimental groups (n = 8 rats/group). Group 1 served as the non-diabetic controls (Non-Diabetic Control, Lean) and was fed a standard ad libitum diet (Purina Mills, Brentwood, MO). Group 2 served as the type 2 diabetic controls (Diabetic Control, Placebo) and was fed a standard ad libitum diet (Purina Mills, Brentwood, MO. Group 3 (Diabetic Treatment, NBC) was fed a niacin bound chromium (NBC) enriched food pellet ad libitum (Purina Mills, Brentwood, MO). The amount of niacin-bound chromium supplement the rat consumes is approximately 1.2-1.9 mg per kg body weight. Each group was maintained on their respective diet for a total of 12weeks.
Testing protocol
After the initial 4 day acclimation period, rat body weights were recorded and then taken every twoweeks. At the initiation of the diet, blood samples were obtained. This was repeated atweeks 2, 6, and 12. Fasting blood glucose measurements were taken using the Accu-Check (Roche Diagnostics) blood glucose monitoring system. Relative renal function (plasma creatinine) was determined by using a plasma creatinine assay kit (Cayman Chemicals, Ann Arbor, MI).
Post-euthanasia analysis
At the end of the 12week period, rats were euthanized and one kidney was removed then the renal medulla and renal cortex were separated, collected and stored in liquid nitrogen for renal eNOS and nNOS protein level analyses by Western blot.
Western blot Analyses of eNOS and nNOS
Western blots were performed to assess the kidney cortex and medulla protein levels of eNOS and nNOS isoforms. Frozen kidney samples were homogenized in HEPES/sucrose buffer containing proteinase inhibitors and centrifuged at 8000g for 20min at 4°C. Electrophoresis was performed with unboiled samples at 4°C on 5-15% Tris-HCl SDS-PAGE gradient gels. Total protein concentration was determined for each sampled supernatant using a Bicinchoninic.
Weight gain
The three groups of rats gained weight at different rates. There were no significant differences in weight gain among the rats. The NBC treatment group, overall, had lower body weights than the Diabetic control group, forweeks 4-12. The Lean control group had the lowest body weight for all time points (Figure 1).
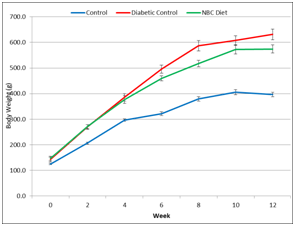
Figure 1 Effect of Niacin-bound chromium supplementation on weight gain over 12 weeks. Data are represented as means ± standard deviations.
Fasting blood glucose
Glucose levels in the NBC treatment group were significantly higher atweeks 0 and 12 than those in the Non-Diabetic Control rats and significantly lower than in the Diabetic Control rats atweeks 2 and 6. Fasting blood glucose levels in the Diabetic Control rats were higher at allweeks than those in the Non-Diabetic Control rats. NBC supplementation did improve fasting blood glucose levels after initiation of diet, but did not improve fasting blood glucose levels at the termination of the experiment (i.e., 12weeks) (Figure 2).
Plasma creatinine
Plasma creatinine levels in NBC rats were significantly lower atweek 6 andweek 12 than those in the Non-Diabetic Control rats and in the Diabetic Control rats (Figure 3). Plasma creatinine levels in the Diabetic Control rats were higher at allweeks than those in the Non-Diabetic Control rats. NBC supplementation did improve plasma creatinine levels after initiation of diet and throughout the 12weeks of feeding (Figure 3).
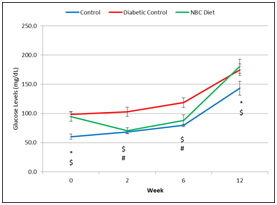
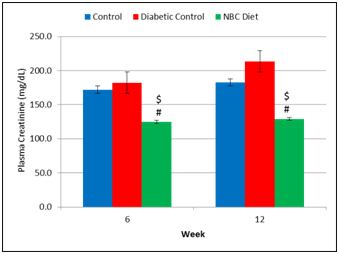
Figure 3 Effect of Niacin-bound chromium supplementation on plasma creatinine levels over 12 weeks. Data are represented as means ± standard deviations. Non-Diabetic Control versus NBC: $ p < 0.05; Diabetic Control versus NBC: # p < 0.05.
eNOS and nNOS protein levels
In our experiment we was observed 74, 77, and 130 kDa eNOS splice forms. The induction of diabetes was associated with a significant decrease of the 74 and 130 kDa isoforms and total amount of eNOS in the kidney cortex and the74 kDa eNOS splice form in the rentel kidney medulla. NBC treatment in cortex resulted in positive but non-significant changes of previously decreased eNOS expression and appearance of additional 77 kDa splice form. The same time it was observed normalization of 74 kDa eNOS splice form in medulla (Table 1). In the case of nNOS, We observed the presence of the 73, 136, and 152 kDa splice forms. Diabetic Control animals displayed a significant decrease of the 73 and 136 kDa and total amount of nNOS in the rentel cortex. Whereas the 152 kDa isoform was not detected. In Diabetic Control animals there was only a decrease 73kDa nNOS isoform. NBC treatment induced a further decrease of all detected nNOS splice forms (Table 2).
Non-Diabetic Control |
Diabetic Control |
Diabetic +NBC |
|
Cortex |
|||
74 kDa |
3.727±0.502 |
0.734±0.103* |
1.597±0.190* |
77kDa |
Not detected |
Not detected |
1.213±0.196 |
130 kDa |
2.757±0.707 |
0.564±0.191* |
0.798±0.065* |
Total |
6.485±1.209 |
1.298±0.282* |
3.457±0.288* |
Medulla |
|||
74 kDa |
2..932±0.958 |
0.913±0.066* |
1.786±0.424 |
130 kDa |
1.532±0.586 |
0.921±0.083 |
1.066±0.349 |
Total |
4.464±1.507 |
1.834±0.148 |
2.853±0.743 |
Table 1 Effect of Niacin-bound chromium supplementation on eNOS/β-actin ratio in the kidney cortex and medulla (*p<0.05 vs. control)
|
Non-Diabetic Control |
Diabetic Control |
Diabetic +NBC |
Cortex |
|||
73 kDa |
1.548±0.135 |
1.018±0.114* |
0.132±0.014*# |
136 kDa |
1.049±0.203 |
0.539±0.119* |
0.054±0.014*# |
152 kDa |
0.018±0.005 |
Not detected |
Not detected |
Total |
2.616±0.328 |
1.557±0.206* |
0.274±0.037*# |
Medulla |
|||
73 kDa |
1.411±0.140 |
1.040±0.083* |
0.099±0.032*# |
136 kDa |
0.646±0.075 |
0.918±0.134 |
0.043±0.012*# |
Total |
2.057±0.204 |
1.958±0.210 |
0.142±0.034*# |
Table 2 Effect of Niacin-bound chromium supplementation on nNOS/β-actin ratio in the kidney cortex and medulla (*p<0.05 vs. control, #p<0.05 vs. high sucrose diet)
Dietary NBC supplementation given to type 2 diabetic rats preserved renal function compared to type 2 diabetic rats’ not receiving NBC supplementation. When NBC was supplemented to the diet of type 2 diabetic rats, it enhanced the activity of NO via NOS. NO is a highly reactive free radical species, generated by the enzyme, NOS.5 It is believed that the NO produced, is then activated via eNOS into guanylyl cyclase (GC). GC is then transformed into cyclic guanosine monophosphate (cGMP) via the biosynthetic pathway17 owing to the rise of NO and eNOS, there were increased levels of the second messenger cGMP. The increased cGMP acts on the vascular endothelium, resulting in vasodilation, which then raises the GFR. Another interesting observation is the association with the NOSs, nNOS and eNOS. Increased vasodilation of efferent arterioles and decreased vasodilation of afferent arterioles leads to increased GFR. Greater vasodilation slows the progression of renal failure and diabetic nephropathy by maintaining renal blood flow.18 It is speculated that renal vasodilation most likely occurred in this study due to the observed greater levels of mediators involved in the NO biosynthetic pathway. Estimation of GFR based on plasma creatinine is a practical, clinically relevant approach to assessing kidney function in people with T2DM. Plasma creatinine levels are typically higher in type 2 diabetic patients than non-diabetic patients.19 Low plasma creatinine levels indicated increased filtration for those diabetic rats supplemented with NBC. The NBC-treated diabetic rats had lower plasma creatinine levels throughout the entire experimental period when compared to both non-diabetic and diabetic control rats. Therefore, low plasma creatinine levels showed increased filtration, and presumably increased GFR, for those diabetic rats supplemented with NBC. Once the T2DM diagnosis is made, a dietary NBC supplementation used as an early intervention may prove to be beneficial in these patients. Increased levels of eNOS and lower levels of nNOS in the renal medulla and cortex were detected in the NBC-supplemented rats compared to the diabetic control rats. eNOS causes positive activation of NO leading to cGMP and vasodilation.20 nNOS-derived nitric oxide plays a role in the control of systemic and renal hemodynamics in normal and diabetic rats. Diabetic rats have shown increased activity of this pathway in the diabetic kidney.11 It is important to note that in the normal, non-diabetic kidney, nNOS is prevalent but does not have much of a biological role. In a diabetic kidney, nNOS leads to increased vasoconstriction. Therefore, it is to be expected to observe low levels of nNOS in the NBC-treated rats when compared to both non-diabetic control and diabetic control rats, as can be seen in this experiment.
NBC can ameliorate hypertension, dyslipidemias and diabetes mellitus, and may be useful in weight management. There is evidence to suggest that chromium supplementation may improve insulin sensitivity and glucose metabolism in patients with glucose intolerance.12 Numerous non-drug substances, such as trivalent chromium, have been shown to influence the glucose-insulin system in a favorable manner. Importantly, most natural substances that affect the glucose-insulin system work satisfactorily over long periods of time and are safe enough to be used chronically. Natural alternatives could prove useful to ameliorate or even prevent the development of insulin resistance.21 In our experiment we observed a beneficial effect on the preservation of glomerular filtration rate, via lowered plasma creatinine levels, increased eNOS, and inhibition of nNOS, in our diabetic model throughout the study.
Fasting blood glucose levels were significantly lower in the NBC-treated rats compared to the Diabetic Control rats at weeks 2 and 6. Atweek 12, the NBC-treated rats had similar fasting blood glucose levels when compared to the Diabetic Controls. The Diabetic Control rats had significantly higher fasting blood glucose levels at all experimental times when compared to Non-Diabetic Control rats. In one study, no effect of NBC supplementation was observed on fasting blood glucose levels.22 An oral glucose tolerance test (OGTT) revealed a significantly improved clearance of blood glucose between 1 and 2h of glucose challenge in NBC-supplemented mice.22 Therefore, an OGTT should be performed to confirm the implications shown from fasting blood glucose. Body weight measurements resulted in the Diabetic control and NBC-treated groups gaining weight when compared to control rats. Although the NBC treated rats weighed less at all experimental times, they only weighed significantly less at weeks 8 and 12 when compared to diabetic rats, suggesting there is a correlation between duration of treatment and administered NBC. Together, these findings imply that NBC supplementation has beneficial effects in preserving renal function in type 2 diabetic subjects when implemented at the initial time of diagnosis. In future studies, alterations in the NO pathway need to be determined. To further support this study’s findings, creatinine clearance (urine and plasma creatinine levels, urine output, and time), plasma cGMP, and OGTT should be tested. Also, the different stages of diabetes and an extended experimental timeline should be considered. Early biopsy of the kidney might be an indicator of the severity of the progression of diabetic nephropathy, before renal failure, and therefore provide further insight into the timing of early treatment to slow its progression. The inexpensive dietary NBC supplementation could be considered a nutritional supplement administered to type 2 diabetic patients, as an alternative to other available anti-diabetic drugs.
Niacin-bound chromium may be an inexpensive alternative for type 2 diabetics. In this study, early intervention with Niacin-bound chromium supplementation was beneficial by preserving renal function, presumably via increased eNOS levels leading to renal vasodilation. Also, renal function and hemodynamics have been shown to be enhanced when nNOS is inhibited, as was observed in this study since significantly decreased levels of nNOS was found in the NBC-supplemented rats. Marked improvements were also seen in body weight and fasting glucose with supplementation of NBC. Additional studies are needed to examine the many mediators involved in the nitric oxide pathway, renal function throughout the experimental time frame, and the effects NBC has on insulin resistance to further support this claim.
The authors would like to acknowledge that this study was funded by the Ohio University Heritage College of Osteopathic Medicine Centers for Osteopathic Research and Education.
Author declares that there is no conflict of interest.

©2016 Wood, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.