MOJ
eISSN: 2471-139X


Research Article Volume 2 Issue 3
1Department of radiology, Tabriz University of Medical Sciences, Iran
2Department of Anatomy, Urmia University of Medical Sciences, Iran
Correspondence: Mohammad Amin Dolatkhah, Department of Anatomy, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran, Tel +984133826965, Fax +98-4412780800
Received: April 15, 2016 | Published: April 25, 2016
Citation: Ghavami SM, Dolatkhah MA, Farjah GH. The effect of chick embryo cerebro-spinal fluid in microwave irradiated collagen guide channel on sciatic nerve regeneration in rat. MOJ Anat Physiol. 2016;2(3):77–80. DOI: 10.15406/mojap.2016.02.00047
The aim of this research was to evaluate the effect of chick embryo cerebro-spinal fluid (e-CSF) across the irradiated collagen guide channel on sciatic nerve regeneration in comparison with autograft. Forty adult male Sprague-Dawley rats (250-300g) were randomized into (1) collagen channel + E-CSF, (2) collagen channel + normal saline (NS), (3) autograft (AG), and (4) sham surgery groups. The left sciatic nerve was exposed and a 10mm nerve segment was cut and removed. In the collagen groups, the proximal and distal cuts ends of sciatic nerve were telescoped into the nerve guides and E-CSF or NS injected into collagen conduit. To the autograft group, the 10 mm nerve segment was turned backwards and used an autologous nerve graft. All animals were evaluated by sciatic functional index (SFI) and electrophysiology testing. The improvements in SFI since the beginning of the last evaluation in experimental groups were measured. On days 49 and 60 post-operation, the mean SFI of the collagen + E-CSF group was significantly greater than the autograft group (P<0.05). On day 90, the mean nerve conduction velocity (NCV) of the collagen + CSF group was greater than autograft group (P<0.05). These findings showed that RPS in collagen nerve guide channel effectively enhances nerve regeneration and promotes functional recovery in injured sciatic nerve of rats.
Keywords: embryo cerebro-spinal fluid, collagen conduit, nerve regeneration
AG, autograft; SFI, sciatic functional index; CSF, cerebro spinal fluid; NCV, nerve conduction velocity; NGC, nerve guidance conduit; NGF, nerve growth factor; TGF, transforming growth factor; EGF, epidermal growth factor; NS, normal saline
Peripheral nerve injuries lead to significant loss of neuron function, and the first choice for treating severe nerve defects involves implantation of autografts to bridge the defect in the injured nerve site. However that is limited by graft availability, secondary deformities, potential differences in tissue structure and size.1 Novel approaches attempt to provide an alternative to autologous nerve graft. Nerve guidance conduit (NGC) have been designed to bridge the gap and provides guidance for sprouting axon and provide a place for diffusion of neurotropic factors and endogenous/transplanted cells at the lesion site.2 It is believed that an ideal nerve conduit should provide not only structural support for damaged nerve but also the neurotropic and neurotrophic support for axonal regrowth.3 Collagen biomaterials were found to be highly biocompatible, absorbable and able to promote Schwann cell adhesion, proliferation and migration.4 Collagen nerve conduits have been shown to be effective in rat and monkey models of nerve repair and are FDA and EU-approved for clinical application.5 Lohmeyer et al. (2007) used collagen conduits to repair sensory nerve avulsions in 11 patients and observed good improvement in nerve recovery. Numerous experiments indicated that, after nerve injury, cell adhesion molecules and neurotropic factor played very important roles in nerve regeneration, cellular viability, growth, activity and functional recovery.6 Among the neurotrophic factors, nerve growth factor (NGF) plays a critical role. Supplementing the nerve site with NGF has been shown to enhance proliferation and promote nerve repair following injury.7 A number of investigators reported Embryonic CSF (E-CSF) contains high concentrations of NGF, transforming growth factor-α (TGFα), transforming growth factor-β (TGF-β), epidermal growth factor (EGF), and neurotrophin-3 (NT-3).8 Early experiments on rat embryos have shown E-CSF supports viability and proliferation of cortical cells in vitro.9 Gato et al.10 were cultured mesencephalic neuroectoderm for 24hr with defined culture medium supplemented with chick E-CSF, show that E-CSF promotes neuroepithelial stem cell survival, and induces proliferation and neurogenesis in mesencephalic explants. Martin et al.11 identified several isoform of FGF-2 in CSF during early stages of development in chick embryos and demonstrated that E-CSF has an effect on the regulation of neuroepithelial cell behavior, including cell proliferation and neurogenesis.11 In the present study, our purpose was to evaluate the effect of collagen tube containing chick embryo cerebro-spinal fluid on repair of a 10mm sciatic nerve defect in rats.
Animals
Forty adult male Sprague-Dawley rats (250-300g) were randomized into:
The Tabriz University of Medical Sciences Ethical Committee approved all experiments.
Preparation of chick embryo cerebro-spinal fluid
Fertile chick white Leghorn eggs were incubated at 38±1°C in a humidified atmosphere. CSF carefully aspirated under a microscope from incubated chick embryos in day E17 by using a 20µL pulled tip glass microcapillary pipette, which was held steadily inside the brain ventricles. The maximum amount of CSF collected from each embryo was 15µL. E-CSF was collected from 30 chick embryos according to development stage based on Hamburger and Hamilton (1951). CSF samples were kept at 4°C during collection to minimize protein degradation. The aliquots were centrifuged at 15,000 rpm at 4°C for 10minutes to remove any contaminating cells.8 None of the samples used in this experiment had any visible sign of contaminating red blood cells.
Preparation of collagen tube
Type-І Collagens were extracted from rat-tail tendons by modification of the previously published method.12 In brief, rat-tail teased out from 3month-old rats and washed in ethanol 70 °C for 45min. Tendons were excised from rat-tails, washed twice with PBS, and then soaked in distilled water and 4mmol/L acetic acid. After stir for 7 d at 4°C. The mixture was decanted into 50ml Falcon tubes and centrifuged at 30000 g for 30min. The supernatant, containing the collagen-I that was stored in sterile universal bottles in 15ml aliquots at 4°C until use. Collagen protein concentration was measured by dry weight and averaging the concentration values by evaporating out the solvent from 0.25, 0.5, and 1.0ml samples in a 110 °C oven for 2h. In order to sterilize the collagen I, chloroform (10% of the volume of collagen) was added to bottom of the bottle beneath the collagen-I solution and allowed to rest overnight at 4°C.13 We then aseptically removed the collagen solution. These solutions were mixed with distilled water, NaOH and phosphate buffered saline of pH 7.4 for a period of 30min, then the mixture was placed into a dish under laminar airflow at an ambient temperature of 25_C and then removed from the dish under aseptic conditions.14 The collagen film was taken and rotated over the Teflon mandrel (1.6mm) manually under sterile conditions to a longitudinal orientation as reported earlier. The Teflon mandrel along with the collagen tube was removed and was subjected to air-drying in a sterile laminar flow hood. The tubes were then removed and washed exhaustively with double-distilled water for a period of 3h under agitation. The tubes were cross-linked using glutaraldehyde (GTA) (0.03%) in PBS of pH 7.4 for 30minute15 and irradiated with microwave irradiation (MWI) (30s each with intermittent cooling time of 2min.15 Tubes were then exhaustively washed with double-distilled water and dried under sterile conditions.
Surgical procedure
Animals were anesthetized with intraperitoneal injection of Ketamine (90mg/kg) and Xylazine (10mg/kg). The left hind limb was disinfected, shaved and prepped. The left sciatic nerve was exposed through a 3-4cm longitudinal gluteal muscle splitting incision on the lateral side of the left thigh. The sciatic nerve was exposed and dissected free from the underlying muscle bed. In the sham operation, the entire left sciatic nerve was exposed and carefully sutured. A 10mm sciatic nerve segment was then transected at the mid tight level and removed. In the collagen group, nerve conduit, both the proximal and distal stumps of the sciatic nerve, were inserted into the lumen ends of the nerve guides and fixed in place with a single 10-0 nylon epineurial suture. After placement lumen in the collagen+, E-CSF and collagen +NS groups were filled with e-CSF and normal slain. In the Autograft group, 10mm nerve segment was turned backwards and used as an autologous nerve graft. On both proximal and distal stumps, single 10-0 nylon epineurial sutures and small gauge needle were used. The muscle border was approximated with 4-0 Dexon sutures and the skin incision were closed with 3-0 nylon. The entire experiment was performed under aseptic conditions using under operating microscope. After surgery, animal was observed and its body temperature maintained with a heating pad until it awoke and were placed in individual cages with free access to food and water ad libitum and a cycle of 12h light/12hr dark.
Functional tests
To determine the sciatic functional index (SFI), walking track analysis was taken on the day prior to and the 7th, 21st, 35th, 49th, 60th and 90th days after the operation. Black non-toxic water-soluble ink was applied to the plantar surface of the hind feet to dip all anatomical regions, and the rats walked across a clean sheet of white paper track and leave footprints. The footprints were measured and SFI calculated on both operated and unoperated limbs according to the formula developed by Bain.16
Formula: SFI=-38.3 ((EPL-NPL)/NPL) + 109.5((EPS-NTS)/NTS) + 13.3 ((EIPNIT)/NIT) – 8.8.
SFI 0 and 100 indicate normal function and complete transaction of the sciatic nerve.
Electrophysiological study
At days, 28 and 90-post operation, the rats in each group were anesthetized with an intraperitoneal injection of a mixture of Ketamine (90mg/kg) and Xylazine (10mg/kg). The animals were prepared for surgery and their body temperature was maintained between 36.5-37°C using a temperature thermostatic pad. The left sciatic nerve (operated side) was re-exposed through a longitudinal muscle splitting incision in the mid-thigh at the pervious surgical site. The electrophysiological study was performed by using Narco bio-system (USA). The stimulating electrode pair were placed 20mm apart on each side of the epineurial sutures, and a recording electrode was inserted into the gastrocnemius muscle. The difference in electromyography latency, amplitude and distance between proximal and distal stimulation sites was measured to calculate conduction velocity.
Statistical analysis
Statistical analyses were processed using the statistical SPSS software (version 16.0) and presented as means±standard error of mean. Result were analyzed using one-way ANOVA were computed with 95% confidence intervals using. A post-hoc study was carried out to examine any significant differences between the groups. Statistically significant differences were considered at P<0.05.
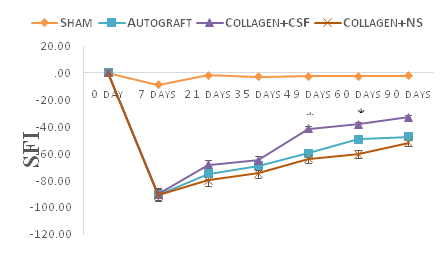
The absence of rat mortality and wound infection postoperatively demonstrated that no wound infections or autophagy occurred. After operation operative procedures were completed, several animal were noted to have ulceration in experimental limbs but these healed without complication. Wounds healed properly, indicating that the operations were well tolerated by the animals. After implantation to repair the defects of the Rat’s sciatic nerves, the tubes were completely resorbed by 12weeks, leaving no residue. Figure 1 shows neurofunctional recovery was assessed by sciatic function index from the day prior to surgery, and then on days 7th, 21st, 35th, 49th, 60th and 90th post-operation. Prior to surgery, SFI values were near zero for all groups. The mean SFI decreased to -100 after the nerve transection due to the complete loss of sciatic nerve function in all animals. The SFI improved from the first to the last evaluation in collagen + E-CSF and autograft groups, since it increased from -90.26±3.6 and -90.53±2.55 during the 7th postoperative day to -32.95±5.50 and -47.44±3.21 during the 90th post-operation day, respectively. On the days 49 and 60 post-operation SFI analyses showed that, neurofunctional recovery was better in the autograft and collagen +E-CSF than collagen+NS group (P<0.05) (Figure 1). On the day 90th, the mean nerve conduction velocities (NCV) were 39.70±0/53 and 30.05±3.72 m/sec for the collagen+E-CSF and autograft groups, respectively. The NCV of the autograft group was faster than that of the collagen+NS group; the difference was statistically significant (P<0.05). The mean amplitude (AMP) for the collagen+E-CSF and autograft groups were 5.72±0/28mV and 4.76±0.98mV, respectively. The difference was not statistically significant (P>0.05) (Table 1).
Group |
NCV (m/s) |
Amp (mv) |
Collagen +E-CSF |
39.7* |
5.72 |
Autograft |
30.05* |
4.76 |
Sham Surgery |
49.6* |
9.7 |
Collagen +NS |
21 |
3.6 |
Table 1 Nerve conduction velocity (NCV) and maximum Amplitude (AMP) comparisons in each group after operation
*P<0.05, the difference sham surgery and Collagen +E-CSF /Autograft were significant. The difference between Collagen +E-CSF and Autograft groups were not significant (P>0.05, One-Way-ANOVA). Results are presented as mean ± SEM.
NCV: Nerve Conduction Velocity; AMP: Amplitude; E-CSF: Embryo Cerebro-Spinal Fluid
Results from this study have clearly demonstrated the E-CSF in collagen guide channel significantly enhances peripheral nerve regeneration in vivo. Attempts to enhance nerve regeneration and promote functional recover of injured nerves is still one of the most challenging issues and a problem to surgeons.17 Artificial nerve conduit to bridge the gap between severed nerve stumps is widely accepted as a useful alternative that provides structural support and a favorable microenvironment for axonal regeneration.18 The great advantage of collagen is very resorbable, and Archibald et al.19,20 described excellent regeneration following median nerve repair in monkeys as by. Its structure also offers biochemical support for nerve regeneration, as it has been showed that collagen is one of extra-cellular matrix (ESM) factors that has been shown to play a significant role in the process of nerve regeneration.6 Collagen nerve conduits degradation speed can be controlled by controlled by manipulating the thickness of the tube and the temperature for thermal dehydration. This condition is essential to permit application of the collagen tube in nerve gaps of different length.19 Chamberlain et al.21 used silicon tubes filled with collagen and show improved regeneration over a 10mm rat sciatic nerve gap compared to empty silicone controls. Our results demonstrate that both collagen and autograft repairs promote nerve regeneration. In this respect, neuromas had not been observed in collagen tube repaired nerves in which regeneration was successful. However, physical nerve guidance by them may not be sufficient to foster optimal nerve regeneration and functional recovery.3 The neurotrophins NGF, NT-3 and BDNF show a well-defined and selective beneficial effect on the survival and phenotypic expression of primary sensory neurons in dorsal root ganglia and of motoneurons in spinal cord following to axotomy.22,23 The remarkable finding of this study was the accelerating effect of chick E-CSF administration on axonal regeneration in rats. Chick E-CSF can be obtained in large quantities and is inexpensive, sterilized, and easily stored. Moreover, the fact that the protein fraction of the CSF is more complex in embryos than in adults, and that it is detected at higher concentrations,10 has led some authors to suggest that the E-CSF is involved in the regulation of neuroepithelial cell behavior. In addition, analysis of E-CSF shows that different mitogenic factors with various concentrations are present in this fluid including: nerve growth factor (NGF),8 insulin like growth factors (IGFs),24 vascular endothelial growth factor (VEGF),25 and transforming growth factor-β1 (TGF-β1).26 In this study. Chick e-CSF was collected from chick embryos at day 17 because of the peak in NGF concentration at days 17 and 18, and IGF-I concentration at day 14 in the embryonic chicken. In addition, Chick E-CSF concentrations of TGF-β1 and VEGF increase from days 6 to 15 and days 6 to 11 respectively.9 NGF is an important neurotrophin that has a role in the survival and proliferation of the cells of the developing nervous system27 while IGF-I plays an important role in the regulation of mammalian growth,28 TGF-β1 regulates the differentiation of neuronal, immune, mesenchymal and epithelial cell types.29 The importance of TGF-β1 signaling has been demonstrated in vascular morphogenesis.30 During peripheral nerve regeneration, TGF-β1 up-regulates beta fibroblast growth factor expression in the anterior horn motoneurons of the spinal cord.31 VEGF administration has also been shown to support and enhance the growth of regenerating nerve fibers, probably through a combination of endogenous, neurotrophic and Neuroprotective effects.32‒34 SFI is a simple and repeatable method for evaluating the functional condition of sciatic nerve injuries. After transection, the SFI value decreased significantly, and NCV could not be detected at first month after surgery. At 90days after operation, the mean SFI between Collagen+E-CSF and Collagen+NS was significant. The SFI value and NCV of group Collagen+E-CSF were to similar to that of group autograft. These finding supports that idea that the combination of SFI with electrophysiological assessment is more comprehensive than electrophysiological method alone.
In conclusion, the present study shows that compared to normal saline, treatment with chick E-CSF in collagen guide channel significantly promote peripheral nerve regeneration.
None.
Author declares that there is no conflict of interest.

©2016 Ghavami, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.