MOJ
eISSN: 2471-139X


Research Article Volume 5 Issue 3
1Department of Veterinary Anatomy, University of Jos, Nigeria
2Department of Veterinary Anatomy, Federal University of Agriculture, Nigeria
Correspondence: Gosomji IJ, Department of Veterinary Anatomy, Faculty of Veterinary Medicine, University of Jos, Plateau State, Nigeria, Tel 2348136070134
Received: January 25, 2018 | Published: June 5, 2018
Citation: Gosomji IJ, Omirinde JO, Hena. Saturated salt solution an alternative reagent in reducing formaldehyde concentration in embalming. MOJ Anat & Physiol. 2018;5(3):205–207. DOI: 10.15406/mojap.2018.05.00192
A well embalmed cadaver for a participatory dissection should be able to have long-term structural preservation of organs and tissues with minimal distortion, deterred from hardening or any form of modification. Six (6) goats meant for students’ dissection practical were utilized for this study in the period of six (6) months. They were grouped into A, B and C based on concentration of formaldehyde in relation to saturated salt solution. Stock solution of saturated salt solution (SSS) was used to make 5% and 10% of formalin used in grouped A and B respectively while group C was 20% formalin with no saturated salt solution. It was observed better range of motion (ROM) on pressure application on joints of the limb in group A and B. The integrity of the muscle was maintained in group B. In conclusion, this study has proven that saturated salt solution (SSS) is the best, affordable and accessible option of reagent in embalming. It maintained the integrity of the tissue without distorting the color, shape and texture of the structure. Also, the hazardous effect of formaldehyde was reduced thereby making dissection convenient to both students and instructors.
Keywords: embalming, cadaver, goats, formaldehyde, saturated salt solution
The process of using reagents to treat both human and animal dead bodies so as to reduce microorganisms, retard decomposition and restore acceptable physical appearance is known as embalming.1,2 This process is necessary in attaining good dissection when studying anatomy where fundamental principles will be taught at the preclinical phase of veterinary and human medical colleges.3,4
A well embalmed cadaver for a participatory dissection should be able to have long-term structural preservation of organs and tissues with minimal distortion, deterred from hardening or any form of modification and also prevented from fungal or bacterial growth.5 If these are achieved in embalmed tissue, curiosity and self-exploratory mindset in dissection with the target of building an in-depth knowledge of anatomy in medical students will be ignited.6,7 Although cadaveric dissection seems to be getting obsolete in some developed countries, some school of thought argued that these ancillaries serve as valuable teaching tools, but cannot replace the hands-on experience that dissection affords.8,9
In developing countries where cadaveric dissection seems to be well utilized for both undergraduate and postgraduate, it is important to provide the cheapest yet a quality embalming fluid. It is also necessary to improve on the type of embalming fluid since most of the reagents have the ability to affect human’s health. The common embalming chemical used is formaldehyde.3 It is a colorless gas with a pungent and irritating odour which could also result to lacrimation when at air concentration.10 Studies conducted by Department of Health and Human Services (DHHS), International Agency for Research on Cancer (IARC) and Environmental Protection Agency (EPA) confirmed that the use of formaldehyde is carcinogenic to both animal and human.10 Therefore an affordable and available alternative is key in embalming processes.
Six (6) goats meant for dissection practical were purchased from a local market in Plateau State, Nigeria. They were grouped into A, B and C having two (2) per group based on different concentration of formalin (Table 1). They were starved overnight to reduce bulk content. The next day, they were administered ketamine via jugular vein. The goats were then placed on lateral recumbency to determine the location of jugular furrow at the neck region. A longitudinal incision of about 5cm was performed along the skin were the jugular vein was located after which a blunt dissection was performed until common carotid artery was located. The artery was then ligated at two (2) points (the upper one towards the head and the lower towards the body) of about 2cm interval. Between the ligatures, an incision was made on the artery at the lateral view. The ligature at the lower point was loosed to ease the passage of catheter.
S/N |
Composition |
Group A (5%) |
Group B (10%) |
Group C (20%) |
1 |
Formaldehyde |
0.5L |
1L |
2L |
2 |
Phenol |
200g |
200g |
200g |
3 |
Glycerol |
500ml |
500ml |
500ml |
4 |
Isopropyl alcohol |
2.5L |
2.5L |
- |
5 |
Salt stock solution |
9.5L |
9L |
- |
6 |
Water |
- |
- |
8L |
Table 1 Composition and quantity of the embalming fluid used in each group
The prepared embalming fluid was infused using a peristaltic pump machine (LongerPump®, BT100-2J made in China) via the incised common carotid artery until the fluid was observed coming out of the extremities.
The cadavers were then kept in the refrigerator at 4°C till after a week which was then subjected to dissection. The dissections were performed for the period of six (6) months. The dissection was approach regionally were photographs taken using Samsung HD Schneider KREUZNACH® 18X zoom, 24 wide angles.
Before and during dissection of each group, parameters such as the general body appearance, movability of the joint, quality of the muscles and organs were observed and recorded. The cadavers in group A and B appeared similar from the 1st month of dissection until the 5th month where the cadavers in group A began to change color and smelling showing signs of deterioration.
External appearance
The range of motion (ROM) on joints of the cadavers in group A and B were observed to be better when little pressure was applied (Figure 1A) while those of group C were observed to be stiff (poor) (Figure 1B). There were no physical changes on the hoof, horns and the furs of the cadavers in all the groups (Figure 1A) (Figure 1B).
Skin folds were observed when the skin of the cadavers in group A and B squeezed with bear hands while C appeared stiffed with no folds. The skin in group A and B appeared soft and easier to incise thus creating a wider incision on the skin (Figure 1C) while in group C it appeared hard and dry with difficulty in incision (Figure 1D).
On the 8th week (2 months) of dissection, the flayed skin and was observed; moist deep fasciae at group A and B (Figure 2) while the deep fasciae in group C appeared dry and hard (Figure 2D).
Muscle quality
On the 16th week (4 months) of dissection, the deep muscles of the thorax observed in group A and B appeared to be moistured, brownish in color and also showing clear demarcation of its boundaries (Figure 3A). Blood vessels, nerves and lymph nodes appeared very obvious (Figure 3C). In group C, the muscles of the deep muscle of the thorax appeared to have dry, wrinkled, pale and brittle. Nerves were not clearly visible and also arteries and veins cannot be easily differentiated (Figure 3B) (Figure 3D).
On the 24th week (6 months) the muscles in group A appeared to lose its color, friable and deteriorated (Figure 4A). The muscles in group B appeared to be relatively soft and plastic while maintaining medium brown coloration (Figure 4B). The integrity of structures such as arteries, veins, nerves and visceral organs were maintained in B was maintained at the 24th week of dissection (Figure 4B). The muscles in group C appeared hard and brittle with pale coloration (Figure 4C).

Figure 1 Day 1: External examination showing an appreciable range of motion in group A and B while in group C it was observed stiff joint (1a and 1b); wide line of incision (black arrow) due to soft and moist skin in groups A and D (1c) while thin line of incision (brown arrow) due to hard and dry skin in group C (1c).
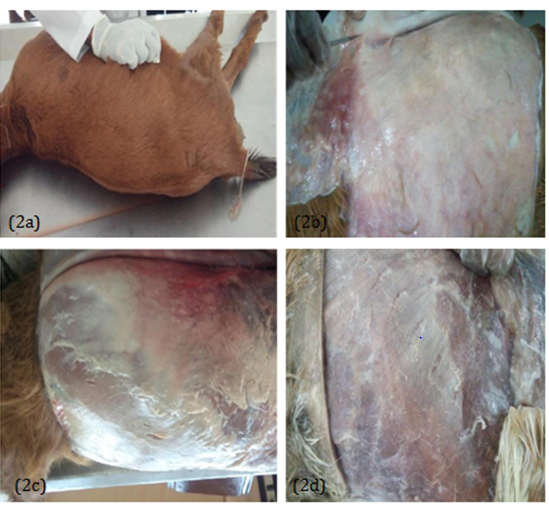
Figure 22 (two) months: Easy to fold skin observed in group A and B (2a); moist deep fasciae in groups A andB (2b and 2c); dry deep fasciae in C (2d).
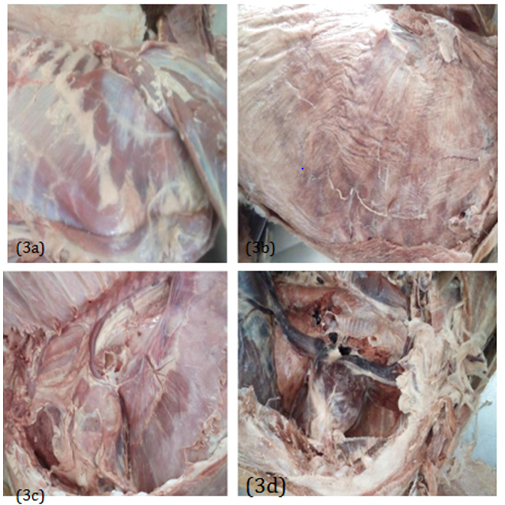
Figure 3 4 (four) months: Deep muscle of the thorax and the thoracic cavity: muscle boundaries and nerves were visible in groups A and B (3a) while in group C there were no visible muscle boundaries and nerves (3b); the thoracic organs appeared fresh and soft in groups A and B (3c) while in group C it appeared dry and hard (3d).
Over the years, efforts were put by researchers in medical colleges to reduce the quantity of formaldehyde in cadavers so as to make dissection convenient and interesting.
Rosenberg & Fitch12 neutralized the effect of formaldehyde in cadavers during dissections by spraying 20% aqueous solution of Infutrace TM and also submerged the cadaver in 10% Infutrace TM in situation where there is strong odor of the formaldehyde. Phenoxetol used as formaldehyde-removing agent drastically reduced irritation and choking smell.13 In this study, the concentration of formaldehyde is reduced by using salt stock solution as the diluent as such there were no irritation and smell at the course of dissection.
Kalanjati et al.,14 observed lighter coloration on the cadaver embalmed with low formalin after six (6) months, this corroborate the findings in this study especially in 5% and 20% formalin. While in 10% formalin the cadaveric coloration was maintained beyond 6 months. It is therefore important to know that the concentration of formaldehyde has effect on the maintenance of the color of cadaver.
An appreciable range of motion (ROM) on application of pressure on the limb of cadavers embalmed in 10% in this study agrees with the finding of Hayashi et al.,15 in human when embalmed with saturated salt solution (SSS). Although the exact mechanism by which the sodium chloride act on the tissue is unclear and no one mentioned its precise action on the tissue.
Unlike studies conducted by Hayashi et al.,15 which conducted bacterial and fungal culture on the embalmed cadaver, in this study there was no test conducted but the purification of the embalmed cadaver at 5% formalin entails the presence of microbes.
The use of saturated salt solution (SSS) to reduce the concentration of formaldehyde in this study has proven to be the best, affordable and accessible option of reagent in embalming. It maintained the integrity of the tissue without distorting the color, shape and texture of the structure. In addition, the hazardous effect of formaldehyde was reduced thereby making dissection convenient to both students and instructors.
The authors of this study declare that they have no conflicts of interest.

©2018 Gosomji, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.