MOJ
eISSN: 2471-139X


Research Article Volume 5 Issue 5
1Department of Medical Laboratory Sciences, Faculty of Basic Medical Sciences, Niger Delta University, Nigeria
2Department of Community Health, College of Health Technology, Calabar, Nigeria
Correspondence: Yibala I Oboma, Department of Medical Laboratory Science, Faculty of Basic Medical Sciences, College of Health Sciences, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria
Received: August 01, 2018 | Published: October 2, 2018
Citation: Oboma YI, Sylvanus B, Okara PN, et al. Protective effect of combined aqueous extracts of Allium sativum and Zingiber officinale against lead acetate induced hepatotoxicity and testicular damage in Rattus norvegicus. MOJ Anat & Physiol. 2018;5(5):306-313. DOI: 10.15406/mojap.2018.05.00215
Background: Allium sativum and Zingiber officinale are medicinal plants used for the management of ailments/diseases and as culinary spices. Their pharmacological potentials ranges from hepatoprotection, immunomodulation, antimutagenic, antioxidant antibacterial, anticarcinogenic, antifungal, hypoglycemic, hyperglycemic and anti-atherosclerotic. However, their synergistic potentials are not fully reported.
Aim: This study was aimed at evaluating the protective potentials of combined aqueous extracts of Allium sativum and Zingiber officinale against lead acetate induced hepatic and testicular damage.
Materials and methods: Albino rats (n=50) were used for this study. The rats were divided into two major groups; acute studies (n=20) and chronic studies (n=30). The acute study was further subdivided into two phases, low dose and high dose of 12 and 8 rats respectively. Calculated doses of lead acetate in mg per kg body weight was given at various concentration of 500mg/kg, 700mg/kg (low dose) and 4000mg/kg, 5000mg/kg and 6000mg/kg (high dose) to determine the LD50. Signs of toxicity and mortality were observed eight hourly and at 24 hours interval. Chronic study (n=30) were further subdivided into 5 (five) groups each containing six (6) animals. Group A received 2ml/kg distilled water and normal feed, Group B (disease control), C, D and E received 2mls of 5000mg/kg body weight of lead acetate only once after 24 hr of starvation, while Group C, D and E were treated (post 72hrs after administration) with 2ml of 1000mg/kg body weight of aqueous extract extracts of Allium sativum, Zingiber officinale and combined extracts of Zingiber officinale and Allium sativum for 4weeks respectively.
Results: Writhing, sedation, swirling, diarrhoea and death were observed at 5000mg/kg and was established as the LD50. Lead acetate induces centrolobular hepatic necrosis and hypertrophy of the seminiferous tubules as revealed by heamatoxylin and eosin stained slides and also elevation of blood Lead level (BLL). Treatment with Allium sativum and Zingiber officinale (combined extracts) showed a statistically significant reduction in blood lead level (t =12.0, p=0.01), and restoration of the hepatic and testicular cytoarchitecture compared to when administered independently.
Conclusion: The study revealed that the synergistic effect of Allium sativum and Zingiber officinale is capable of ameliorating lead acetate induced hepatic and testicular damage as well as reduction of blood lead level in the rats. This synergistic potential may be an excellent reservoir of pharmaceutical and chemical templates for new drugs formulation to be explored.
Keywords: testicular toxicity, hepatotoxicity, Allium sativum, Zingiber officinale
Lead has been described as one of the most toxic metals in our environment.1 It also forms part of a group of metals described as carcinogenic in humans. Its carcinogenic potential is considered to be dependent on the metal's oxidation state, solubility and complex formation.2 Due to increasing anthropogenic activities and vehicular emissions, it has been indicated that the amount of lead available in the environment for potential consumption via food chains and drinking water supplies is on the rise.2 Lead poisoning is also a global health problem but it is unrecognized as such in a number of African countries. Lead poisoning as indicated by elevated blood lead levels (BLL) have been observed in the general population in some parts of Nigeria.3 In adults, occupational exposure to lead is the most common cause of lead poisoning with a wide range of physiological, biochemical, behavioural and organ dysfunctions mostly the kidney, liver, spleen and testes.3 In 2013, lead was believed to have resulted in 853,000 deaths, which occurred mostly in the developing countries with the poor been at greater risk.4 Descriptions of lead poisoning is dated to at least 2000 BC.5,6
Health benefits of Allium sativum (garlic) in lipid regulation, anticarcinogenic, antimicrobial and antioxidant potentials has been extensively reported in previous studies7,8 while Zingiber officinale ginger is arguably one of the most widely used culinary agent and spice in the world.9,10 Phytochemical studies have shown that the unique culinary and medicinal properties of ginger are due to the presence of phytochemicals like zingerone, shogaols, ingerols, pardols, β-phellandrene, curcumene, cineole, geranyl acetate, terphineol, terpenes, borneol, geraniol, limonene, β-elemene, zingiberol, linalool, α-zingiberene, β-sesquiphellandrene, β-bisabolene, zingiberenol and α-farmesene.9,11,12 Scientific studies carried out in accordance with the principles of modern systems of medicine have convincingly shown that ginger possesses numerous health benefits like antimicrobial, antiviral, gastroprotective, antidiabetic, anti-hypertensive, cardioprotective, anticancer and immunomodulatory effects.9,11 Additionally, preclinical studies carried out with laboratory animals have also shown that ginger possesses hepatoprotective constituents, and can protect the liver against the toxic effects of diverse class of xenobiotic agents like alcohol13,14 acetaminophen,15 heavy metals16–18 bromobenzene and lead.19 Several plant species serve as excellent reservoirs of pharmaceutical and chemical templates for new drugs formulation. It is against this background that this study was designed to investigate the protective effects of Allium sativum and Zingiber officinale on lead induced toxicity on the testes and liver of Rattus norvegicus rats.
Experimental animal care and design
Fifty healthy rats of average weight (149-179g) were purchased from the animal house of the Department of Pharmaceutical Sciences, University of Port Harcourt, Rivers State. The animals were randomly divided into two major groups; 20 for acute studies and 30 for chronic studies and housed in cages made of metal nesting and were fed with growers mash and distilled water with 12hours light and dark cycle. The beddings were changed and the cages cleaned every morning and disinfected at interval of three days. The rats were allowed to acclimatize for 14 days before experimentation.
Acute and chronic toxicity study and experimental design
The rats for acute study were divided into two phases. Phase one (low dose) and phase two (high dose) of 12 and 8 animals respectively. Calculated dose of lead acetate in mg per kg body weight was given to the rats at various concentration of 500mg/kg, 700mg/kg (phase one), and 4000mg/kg, 5000mg/kg and 6000mg/kg in phase two. Signs of toxicity and mortality were observed eight hourly and 24hrs interval. Thirty (30) animals were used for chronic study: Five groups each n= 6 rats: Group A (negative control) received 2ml/kg water and normal feed. Group B (disease control), C, D and E received 5000mg/kg body weight of lead acetate only once after 24hrs of starvation. Group C, D and E were treated (post 72 hrs after exposure to lead acetate) with 2ml of 1000mg/kg body weight of aqueous extracts of Allium sativum, Zingiber officinale and combined extracts of Zingiber officinale and Allium sativum for 4weeks respectively.
Extracts and lead acetate preparation
The Zingiber officinale rhizome was purchased in Yenagoa, Bayelsa State. The fresh ginger rhizomes was washed, cleaned, peeled, cut into pieces and dried by hot air oven at 60°C. It was then crushed to fine powder using Electroline blender (Model: CM/L 7962804, China). The aqueous extract was prepared in accordance with the method of Hitesh et al.20 500g of the fine powder was homogenized in 750ml of distilled water and 250ml of ice cold water for 12minutes. The homogenized mixture was filtered using cheese cloth. The filtrate was centrifuged using macro centrifuge at 2500rpm for 10mins, the clear supernatant separated and the volume made up to 1000ml with distilled water. The concentration was regarded as stock (500g/ml) based on the weight of the Zingiber officinale extract. The aqueous extract of Allium sativum was prepared in accordance with the method of Mathew et al.41 500g of Allium sativum bulbs were crushed and added to 100ml of distilled water. The juice was extracted using an Electroline blender (model no CM/L 7962804, China). The mixture was filtered thrice using cheese cloth and then centrifuged using macro centrifuge at 2500rpm for 10mins. The supernatant was transferred to a clean bottle and stored at 2-8°C until use. The concentration was considered 500g based on the weight of the paste/ml. Equal volume of both extracts was regarded as supplement and thus served the animals for the period of experiment.
About 5000mg of lead acetate powder was weighed using Medfield electronic balance and dissolved into 500mls of distilled water and made up to 1000ml. The mixture was stirred using a glass rod until it was completely dissolved.
Extract administration and sub-acute toxicity study
The administered dose was calculated from the stock lead acetate and given as milligram per kilogram body weight. 2ml of the extract was administered orally once a day using metal cannula attached to 2.0ml syringe and lasted for 4 weeks. All the groups were pre exposed to 5000mg per kilogram body weight of lead acetate except control group. The animals were deprived of food and water for 24hrs before administration of the supplement and thereafter allowed access to food. The study was conducted in accordance with the Institute of Health guide for the care and use of laboratory animals 32 as well that of Niger Delta University, Wilberforce Island Bayelsa State.
After 4 weeks of treatment with extracts, the Rattus norvegicus were anaesthetized using light dose of diethyl-ether prior to sacrifice and blood samples collected by cardiac puncture using needle and syringe. Blood samples were transferred immediately to lithium heparin container while the tissues collected were fixed immediately in 10% formal saline for histopathological analysis.
Determination of blood leads level (BLL) and histopathology
Determination of blood lead level was by lead digestion method. About 5ml of concentrated HNO3 and 10ml of concentrated H2SO4 was added to the blood sample gently and carefully. The mixture was heated and cooled intermittently, discharging brown fumes of nitric oxide. Again 5ml of concentrated HNO3 was added and reheated until the solution was clear. It was then made up to 50ml with distilled water and filtered using cotton wool to remove filtrate. The level of lead (Pb) in µg/dl in the blood was determined by atomic absorption spectrophotometer at 217nm wavelength.
The principle of reaction was based on the chemical theory of dye, where the acidic and basic dyes stain the basic and acidic components of the tissue respectively. Heamatoxylin is a basic stain which stains the nucleus which is acidic while eosin is an acidic stain that counter stains the cytoplasm which is basic giving a pink color. Tissue processing was in accordance with standard histological processing schedule by Aviwioro et al.21 The tissues were sectioned at 4µm using rotary microtome and were stained by Erhlich’s heamatoxylin and Eosin. Photomicrographs of the stained tissue sections were produced using a digital microscope (Olympus®) at x400 magnification.
Acute study
The animals were observed continuously for one hr after administration of lead acetate and hourly intervals for eight hrs then after 24 hrs for signs of toxicity such as behavioral changes and mortality. In Phase 1; only behavioral changes was observed while in Phase 2, both behavioral changes and mortality was observed. LD50 was estimated as a cut-off value since no mortality occurred even at values above 5000 mg/kg.
Weight changes
Testis: Acute toxicological study of different concentration of lead acetate on rats revealed increase in the weight of the testis from lower concentration to higher concentration as shown in Table 1 & Table 2.The substance is suspected to have induced testicular hypertrophy in the rats. Following aqueous extracts administration, apart from the group B that had only lead (1.09±0.271) the rats recorded reduction in testicular weight compared with control (1.21±0.40). Whereas, there was increase in testicular weight in groups (C and D) that received Allium sativum and Zingiber officinale extracts respectively. In Group E (1.21±0.60) rats that received combination of both extracts showed testicular mean weight similar to the control group indicating a protective effect.
Liver: Acute toxicological study of different concentrations of lead acetate clearly reveals a reduction in the weight of the rat’s liver displaying a classical reduction in organ weight from 6.40±0.23 in 500mg/kg concentration to 5.86±0.08 in 4000mg/kg a and drastic reduction of weight at highest concentration of 5000mg/kg (1.47±0.47). There was hypertrophy of the liver following lead inducement and subsequent treatment with Allium sativum, and Zingiber officinale extracts to groups B, C, D and E respectively, compared with control group (group A). The liver of group B compared with group A (5.32±0.64 vs. 4.73±0.33) showed an increase in liver weight. The combine hepatoprotective effect of Allium sativum and Zingiber officinale extracts (5.32±0.64 vs. 5.10±0.59) was higher compared with Allium sativum (5.32±0.64 vs. 5.47±0.82) and Zingiber officinale (5.32±0.64 vs. 5.20±0.05) administered independently. The analysis of Variance (ANOVA) of difference in weight among the groups showed a non-statistically significant relationship as well as the post hoc analysis using Turkey method of comparison of variance. Generally, there was statistically significant increase (P≤0.05) in the mean body weight of rats in all the groups as presented in Table 3
Effect of combined extracts on blood lead level (BLL) and histological examination
Table 3 shows the effect of combined extracts on blood lead level on lead induced Rattus norvegicus. There was a decrease in blood lead concentration in Rattus norvegicus administered with Allium sativum (group C) post lead induced poisoning (0.44±0.03 vs. 0.39±0.09). There was equally a statistically significant reduction in blood lead level following oral administration of Zingiber officinale. However, group E showed a drastic reduction in blood lead level after administration of Allium sativum and Zingiber officinale combined for 4 weeks (0.44±0.33 vs. 0.03±0.01) and was statistically significant at t=12.0 and P =0.01. (Figure 1 & Figure 2)
Light microscopy examination of Haematoxylin and Eosin stained representative sections of testicuar tissues and liver tissue as presented in Figure 3 & Figure 4 showed the normal control rat’s liver showing a central vein with normal sinusoids and normal space of Disse. The hepatocytes are normal, all suggestive of normal liver tissue architecture. Likewise, representative sections of testicular tissue from negative control (normal) indicates normal seminiferous tubules containing different types of germ cells; spermatogonia lying on the basement membrane beneath which are the myofibroblasts, spermatocytes, spermatids and spermatozoa. The interstitial tissues found between seminiferous tubules contain interstitial cells and Leydig Cells. Liver tissue section intoxicated with 2mls of 5000mg per kilogram body weight of lead acetate to induce hepatotoxicity shows inflammatory cells filling the portal and spilling over the surrounding parenchyma. There is also distortion of the space of Disse. The substance is hepatotoxic at that concentration, duration and route of administration. In the same manner, histology of testicular tissue of rats administered with 2ml of 5000mg/kg per body weight of lead acetate solution shows hypertrophy of the seminiferous tubules with increase in tubular diameter.
Hepatocytes induced with 2mls for 24 hrs with 5000mg per kilogram body weight of lead acetate solution and then treated with 2mls of aqueous solution of Allium Sativum for 4 weeks shows centrolobular necrosis (Figure 2), swollen hepatocytes, central vein and the surrounding hepatocytes at some point appear unremarkable and filled with inflammatory cells (Figure 2; Group H2). From the histology result, it is obvious that Allium sativum alone has no hepatoprotective potentials against lead acetate induced liver poisoning. Also, group of rats induced with lead acetate poisoning and then administered with aqueous garlic extract (Allium sativum) for 4 weeks shows normal testicular tissue with evidence of tissue regeneration and proliferation. This evidence suggests that Allium sativum is more testicular protective than hepatocytes protective.
Liver and testicular tissue of Rattus norvegicus treated with 2mls of aqueous solution of Zingiber officinale for 4 weeks showed necrotic cells (acidophilic bodies) seen as homogenous pinkish structure. Seen also were swollen hepatocytes, sinusoids, presence of mitotic figures and fatty cells. The presence of mitotic figures is suggestive of ongoing reparative process (hepato-regeneration). In a nutshell, ginger has mild hepatocyte protection against lead induced hepatocyte poisoning. Histological picture of testis reveals normal seminiferous tubules and its features. There is also degeneration of the tubules at some point with loss of basement membrane. Substance showed low protection of testicular tissue against lead poisoning.
Liver and testicular tissue of Rattus norvegicus induced with 2mls (3 doses) for 24 hours with 5000mg per kilogram body weight of lead acetate solution and then 2mls of aqueous solution of Allium sativum and Zingiber officinale combined (1:1) for 4 weeks shows well differentiated liver section with a portal tract with normal morphology and few inflammatory cells. The sinusoid and space of Disse is normal with normal hepatocytes compared to the control. Thus the combination of Allium sativum and Zingiber officinale shows hepatoprotective activities against lead acetate induced liver poisoning. While, histology of testicular tissue shows normal seminiferous tubules with intact basement membrane housing the spermatogonia cells and proliferating spermatocytes and healthy spermatids with prominent spermatocytes compared with those on lead only.
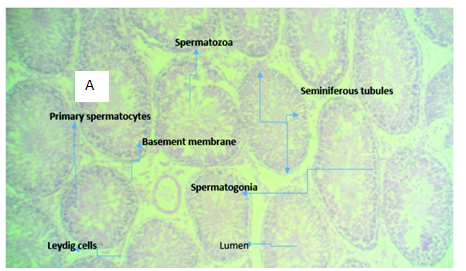
Figure 3A (Control): micrograph of testicular tissue showing normal histomorphology of the testis (normal seminiferous tubules, spermatogonia, spermatocytes).
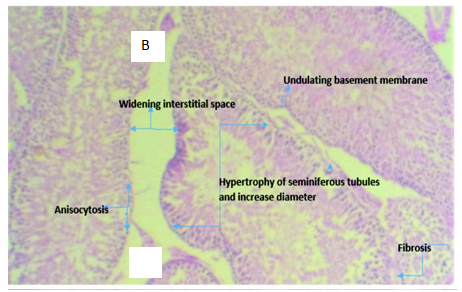
Figure 3B Micrograph of testicular tissue from rat intoxicated with 2ml of 5000mg/kg of lead acetate solution showing hypertrophy of the seminiferous tubules with increase tubular diameter which demonstrates the tissue toxicity potentials of lead acetate
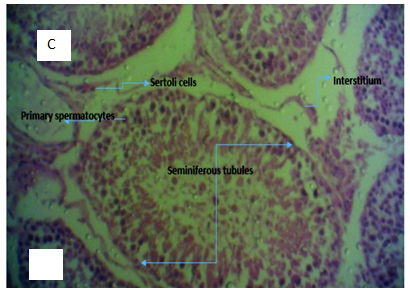
Figure 3C Micrograph of testicular tissue from rats administered with 2ml of 5000mg/kg of lead acetate and garlic (Allium sativum) for 4 weeks showing normal testicular tissue with evidence of tissue regeneration and proliferation.
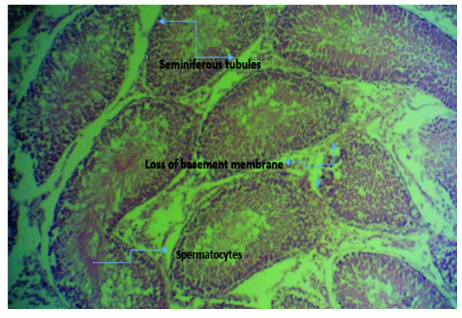
Figure 3D Micrograph of tissue from rat’s testis induced with 2ml of 5000mg/kg lead acetate and then administered with aqueous extract of ginger (Zingiber officinale) for 4 weeks showing normal seminiferous tubules and its features. There is also degeneration of the tubules at some point with loss of basement membrane. Substance showed low protection against lead poisoning.
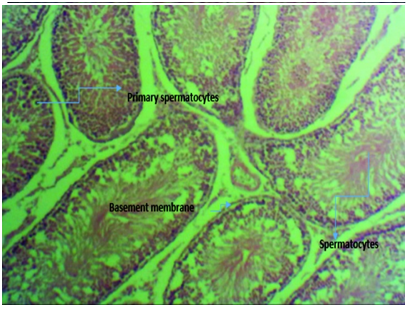
Figure 3E micrograph of rats induced with 2ml of 5000mg/kg lead acetate poisoning and then administered with equal mixture of aqueous extract of ginger and garlic (Zingiber officinale and Allium sativum) combined for 4 weeks showing normal seminiferous tubules with intact basement membrane housing the spermatogonia cells and proliferating spermatocytes and healthy spermatids with prominent spermatocytes compared to those induced with lead without treatment.
Figure 3 Photomicrographs of testicular tissue sections of Rattus norvegicus stained with H & E, with x400 magnification.
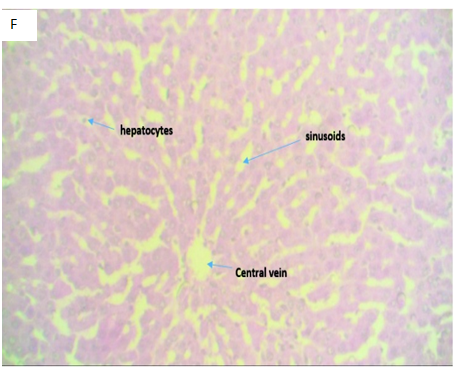
Figure 4F(Control): Micrograph of normal liver tissue displaying a central vein with normal sinusoids and space of Disse. The hepatocytes appear normal, suggestive of a normal liver tissue.

Figure 4G Micrograph of liver tissue of Rattus norvegicus induced with 2mls for 24 hrs of 5000mg/kg body of lead acetate solution showing inflammatory cells filling the portal tract; indicative of lead acetate induced tissue insult.

Figure 4H1 and H2 Micrograph of a liver tissue of Rattus norvegicus induced with 2mls (3 doses) for 24 hrs with 5000mg/kg body weight of lead acetate solution and then 2mls of aqueous solution of Allium Sativum for 4 weeks showing Centro lobular necrosis (H1), swollen hepatocytes, central vein and inflammatory cells (H2). The histology result reveals that Allium sativum has no hepatocytes protection against lead acetate induced liver poisoning.

Figure 4I Micrograph of liver tissue of Rattus norvegicus induced with 2mls of 5000mg/ kg of lead acetate solution for 24 hrs and then 2mls of aqueous solution of Zingiber officinale for 4 weeks showing necrotic cells (acidophilic bodies) appearing as homogenous pinkish structure; swollen hepatocytes, sinusoids and presence of mitotic bodies and fatty cells. The presence of mitotic bodies is suggestive of the reparative and hepatoprotective potentials of ginger.
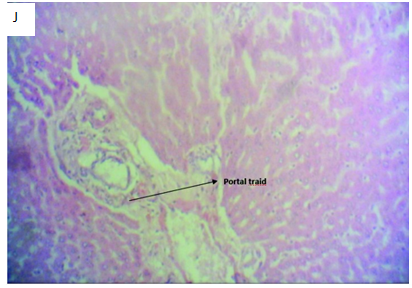
Figure 4J Micrograph of liver tissue section of Rattus norvegicus induced with 2mls of 5000mg/kg of lead acetate solution for 24 hours and then treated with 2mls of aqueous solution of Allium sativum and Zingiber officinale combined (1:1) for 4 weeks showing well differentiated liver section with a portal tract with normal morphology and few inflammatory cells. The sinusoid and space of Disse is normal with normal hepatocytes compared to the control. The combination of Allium sativum and Zingiber officinale shows hepatoprotective activities against lead acetate induced liver poisoning.
Figure 4 Photomicrographs of H&E stained liver tissue sections with x400 magnification.
|
Dose |
Writhing |
Sedation |
Swirling |
Diarrhoea |
Death |
Phase 1 |
Control |
0/4 |
0/4 |
0/4 |
0/4 |
0/4 |
|
500mg/kg |
0/4 |
0/4 |
0/4 |
0/4 |
0/4 |
|
700mg/kg |
2/4 |
¼ |
0/4 |
0/4 |
0/4 |
Phase 2 |
Control |
0/2 |
0/2 |
0/2 |
0/2 |
0/2 |
|
4000mg/kg |
½ |
½ |
0/2 |
½ |
0/2 |
|
5000mg/kg |
2/2 |
2/2 |
2/2 |
2/2 |
2/2 |
|
6000mg/kg |
2/2 |
2/2 |
2/2 |
½ |
2/2 |
Table 1 LD50, 5000mg/kg; numerator, number of rats affected; Denominator, number of animal tested
Organ weight |
|
Concentration of lead acetate |
|
|
500mg/kg |
700mg/kg |
4000mg/kg |
5000mg/kg |
|
Testis (1.21±0.40) |
2.06±0.01 |
2. 07±0.01 |
2.08±0.02 |
2.04±0.05 |
Liver (4.73±0.33) |
6.40±0.23 |
5.47±0.34 |
5.86±0.08 |
1.47±0.47 |
Table 2 Acute toxicological study of oral administration of lead acetate on organs weight (testis and liver) of Rattus norvegicus
Values expressed as Mean±SD, Kg, kilogram; mg, milligram
Variable |
Mean |
SEM |
t-test |
P<0.05 |
comment |
Group B |
0.44 |
0.03 |
|
|
|
Group C |
0.39 |
0.09 |
0.43 |
0.33 |
NS |
Group D |
0.63 |
0.03 |
3.7 |
0.01 |
S |
Group E |
0.03 |
0.01 |
12 |
0.01 |
S |
Table 3 Evaluation of the effect of aqueous extracts on blood lead level of Rattus norvegicus using mass absorption spectrophotometric assay
SEM, standard error of mean; S, significant at P<0.05; NS, non-significant
Group B, lead administered group only
Group C, Lead acetate and Allium sativum
administered grouprGroup D, Lead acetate and Zingiber officinale administered group
Group E, Lead acetate with Allium sativum+Zingiber officinale administered group
The use of medicinal herbs and plants in the management of ailments and diseases is dated back to antiquity. Result oriented biological and pharmacological properties have long been explored in Allium and Zingiber genera most notably; Allium sativum and Zingiber officinale species. Spermatogenesis is a complex process where clinical and pathological manifestations affect fertility. Due to high concentration of polyunsaturated fatty acids within the plasma membrane of free radicals; high levels of it can damage the membrane of spermatozoa which in turn impairs motility of the semen and overall spermatogenesis.11,22 Our results shows that lead acetate is toxic to rat's testis cytoarchitecture as it induces hypertrophy of the seminiferous tubules, widening of the interstitial space thus impairing Leydig cells growth; and this is in agreement with previous studies on the effect of lead on testicular tissues.
Lead was reported to have a cytotoxic effect on the interstitial Leydig cells which secrete testosterone and inhibits the expression of certain enzymes involved in the biosynthesis of steroid hormones.23 Hypertrophy of the seminiferous tubules in group of rats administered with lead acetate was reported in the present study. However, Al-Omar et al.24
and Corpas et al.25 reported that lead causes decrease in seminiferous tubules diameter as well as thickness in adult rats. Garu et al. 26 also observed that different doses of lead impaired the development of various components of the testis and decreases the size of the tubules which is in agreement with the current study that have reported a decrease in testicular weight in rats that received only lead acetate. It is imperative to critically monitor drug-induced changes in animal weight which is an indicator of drug effect according to Nwidu et al.27 Allium sativum is considered as a protective regime for different tissues against arsenic induced toxicity and has also proved a protective role against testis and spermatogenesis damages in lead intoxicated rats.28 This is in agreement with the present study that has reported testicular cytoarchitecture restoration in rats treated with extracts. Such protection may reduce lipid peroxidation and increase antioxidant defense mechanism. Kamtchouing et al.29 observed an increase in testicular weight after 8 days of garlic administration attributable to gingerol in garlic, while Garu et al.26 observed significant decrease in testicular weight and seminiferous tubular diameter.
Zingiber officinale (ginger) has shown beneficial effects on male reproductive functions as observed in adult male rats; evidenced by the normal seminiferous tubules, spermatozoa and basement membrane and increase testicular weight following the administration of Zingiber officinale in the present study. The increase testicular weight could be traced to increase in androgen biosynthesis. Androgen has shown to be important for the development, growth and normal functioning of the testis. Khaki et al.22 also proven that ginger treatment resulted in increased testicular weight, and increased serum testosterone levels in Wistar rats thus, demonstrating the antioxidant and androgenic properties of ginger. According to Jedlinska-Krakowska et al.30 a couple of research have come up with evidence that diet containing vitamins A, B, C, E and antioxidants are capable of increasing the stability or integrity of the blood testis barrier thus; concealing the sperm DNA (deoxyribonucleic acid) from being attacked by free radicals.
Lead induced tissue toxicity was not only restricted to testicular tissue only but also causes hepatotoxicity as evidenced in this study. Previous reports have shown the liver to be the largest repository of lead among soft tissues followed by the kidney.31 The present study observed massive recruitment of inflammatory cells filling the portal tract, swollen hepatocyte and sinusoids following lead inducement. This is in agreement with previous work by Verheij et al.32 which reported the accumulation of significant amounts of lead in the liver resulting in oxidative stress with resultant hepatotoxicity. This study also observed hepatocyte proliferation, fatty changes, hydropic degeneration, and necrosis of the hepatocytes, mild fibrosis and biliary hyperplasia. Dongre et al.33 proposed that two main factors that contribute to toxicological effects of lead are concentration and duration. This present study also observed a concentration dependent effect of lead toxicity and therefore align with the report of Dongre et al.33 Also, lead induced toxicity in the present study revealed the presence of acidophilic bodies seen as homogenous pinkish structures within the necrotic hepatocytes. This may be due to apoptotic alteration, followed by organelles swelling especially in the mitochondria, endoplasmic reticulum and rupture of lysosomes leading to the amorphous eosinophilic cytoplasm as an initial sign in the sequence of hepatocytes necrosis before shrinking and dissolution of nuclei.34 In this present study, the effect of lead acetate on body weight gain was assessed and the final body weight of the rats intoxicated with lead only was significantly lower than that of the healthy normal group. The obtained results are in agreement with previous report by Allouche et al.35 The decreased body weight may be associated with several factors, one of which is imbalanced metabolism produced by impairing zinc-dependent enzymes which are necessary for many metabolic processes; also lead decreases the level of erythropoietin hormone which has anabolic effect. However, Haouas et al.36 attributed the decrease in weight to malabsorption of nutrients from direct toxic effects of lead on the gastrointestinal tract or by inhibition of protein synthesis.
Although, Sankara et al.37 has proven that Allium sativum preparations exhibit hepatoprotective properties, this study has a divergent view, based on the histological picture of the liver tissue. In this present study, the hepatoprotective effect of Allium sativum was too minimal to restore the liver morphology despite its significant reduction of blood lead level. Pourjafar et al.38 also reported the ability of Allium sativum to reduce the lead levels from the liver, kidney, blood and bone. Also Rodrigo et al.39 carried out a study on the curative effect of Allium sativum on alcoholic liver disease subjects and observed that; liver enzymes levels which were increased before Allium sativum administration significantly decreased after Allium sativum treatment.40–42 Our results reveal that Zingiber officinale is hepatoprotective and concurs with previous studies carried out with laboratory animals which claim that Zingiber officinale possesses hepatoprotective effects and is in agreement with Khaki et al.22 and other previous reports.13,14
Zingiber officinale and Allium sativum aqueous extracts combined has shown a substantial anti-inflammatory and hepatoprotective effect compared to when the extracts were administered separately. The combination of both Zingiber officinale and Allium sativum resulted in significant reduction of blood lead level (BLL) compared with the groups administered with either Zingiber officinale or Allium sativum only. This study also observed that; liver tissue in group induced with lead and treated with aqueous extract of Zingiber officinale and Allium sativum showed higher reduction of inflammatory cells and restored to near normal the tissue architecture of liver hepatocytes.43–45
The results of this study has proven that the combination of Zingiber officinale and Allium sativum was able to reverse lead-induced testicular injuries, minimize cytoarchitectural alterations in the testis and ameliorate hepatotoxicity and liver damage. The histomorphology and biochemical changes in the liver as evidenced by histological micrographs and biochemical parameters all showed protective proofs. Although, there is no work that has evaluated the combine effect of both extracts apart from this present study; it is our opinion that instead of using Allium sativum or Zingiber officinale alone in the management of lead induced testicular injury or hepatotoxicity; the duo (Zingiber officinale and Allium sativum) should be used as a combine therapy which will be more effective. However, the results obtained from this study provide the platform for further investigation of the promising therapeutic potentials of Zingiber officinale and Allium sativum combined in treatment of testicular and liver damage.
None
The authors declare that there is no conflict of interest.

©2018 Oboma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.