MOJ
eISSN: 2471-139X


Opinion Volume 5 Issue 5
Department of Pharmacological and Biomolecular Sciences, Universita degli Studi di Milano, Italy
Correspondence: Roberto Maggi, Department of Pharmacological and Biomolecular Sciences, Universita degli Studi di Milano, Via G Balzaretti, 920133 Milano (MI), Italy, Tel +39 02 50318233, Fax +39 02 50318204
Received: September 27, 2018 | Published: October 5, 2018
Citation: Lombardi A, Maggi R. New insight of medical and molecular farming (MMF): the plants derived drugs. MOJ Anat & Physiol. 2018;5(5):315-317. DOI: 10.15406/mojap.2018.05.00216
engineering plants, bio-products, biotechnology, drugs
The synergic collaboration between science and health policies led to a significant increase in life expectancy and, consequently, in world population (Figure 1). By contrast, fulfilling the needs of this growing population, in terms of quality and quantity of production of medical and pharmaceutical compounds, could become quite difficult. In addition, the outbreak of new infectious diseases, metabolic disorders, the increase of chronic diseases as well as the growing requirement of personalized therapies, will raise the demand for many protein-based medicines.
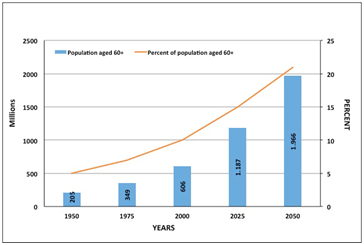
Figure 1 The balance between young and olds. In 2000 about 10% (606 million) of the global population was aged 60+; by 2050 this will be more than 21% (about 2 billion). 75% of people aged 60+ have one chronic condition but 50% have two or more chronic conditions (modified from Rhenu Bhuller –Global contract manufacturing Trends 2010).
Current manufacturing practices are facing a major global capacity shortage for the production of biotechnological drugs and there are fewer than two dozen facilities worldwide capable of large-scale biotech manufacturing. Furthermore, the capacity challenge is also expected to become more complex over the next decades.
In the last years, research has revolutionized the use of valuable proteins for a variety of treatments and their production became possible since several genes can be efficiently expressed in many different biological systems.1 The expression systems so far available offer different advantages for the preparation of the recombinant proteins, in general, the optimal technical approach should guarantee the safety, the biological activity and the economic production. Molecular Farming, is a term indicating the technology for the production of high-value compounds in transgenic animals, and it is nowadays used to refer to the production of pharmaceutically important and commercially valuable proteins in plants.2
Plants have been serving as an extremely useful source for treatments and therapies. Thousands of plant species are historically used to deal with common diseases and most of the world population uses them to cure acute and chronic health problems. After a shift from wild collection to cultivation, advances in plant biotechnology were aimed to direct improvement and modification of specialized constituents of plants like carbohydrates, proteins, oils, fats and vitamins, over a long time.
The use of engineered plants as “bioreactors” for the production of high-value recombinant proteins for industrial and clinical applications has become a promising alternative tool to the conventional bio-production systems, such as bacteria, yeast, and cultured insects and animal cells (Figure 2).3
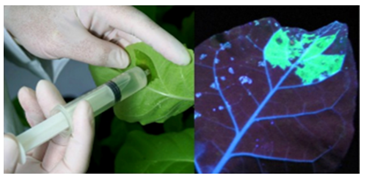
Figure 2 Schematic illustration of expression of transgene in plants. Left panel: infiltration of Agrobacterium carrying the GFP transgene; right panel: visualization of transient expression of the fluorescent protein four days post infiltration examined under a UV lamp.4
What is the idea that withstands on “why to use plants?”
This novel system offers several advantages in terms of safety, quality, production and costs, as summarized in Table 1.
Comparisons |
Transgenic plant |
Bacteria |
Yeast |
Transgenic animals |
Safety |
High |
Low |
Unknown |
High |
Contamination risks |
Low |
Endotoxins |
Low |
Virus, Prions, oncogenic DNA |
Product quality |
High |
Low |
Medium |
High |
Protein folding accuracy |
High |
Low |
Medium |
High |
Glycosylation |
Minor differences |
None |
Incorrect |
Correct |
Protein yield |
High |
Medium |
High |
High |
Production scale |
Worldwide |
Limited |
Limited |
Limited |
Scale-up capacity |
Very high |
High |
High |
Low |
Overall cost |
Very low |
Low |
Medium |
High |
Table 1 Comparison of different expression platforms for the production of pharmaceutical5
Plants are not infected by known human pathogens, such as bacteria, prions or viruses, decreasing the risks of pathogens cross-contaminations and minimizing health dangers. Plants classically produce recombinant proteins with the correct folding activity and glycosylation4 which allow getting high quality recombinant proteins. Plants possess excellent biosynthetic capabilities, including the ability to use the solar energy (photosynthesis) and/or very simple media to support significant biomass and protein accumulation. Finally, they have a large potentiality for a low-cost production as well as easy distribution and storage due to a better stability of plant bio-products.
The use of plants as a production system of pharmacologically active compounds has been widely described in the last years and new evidences on the refining of this technique are ongoing. A few illustrative examples are here reported.
In order to produce oligomer monoclonal antibodies, Huang et al.6 developed a bean yellow dwarf virus (BeYDV) based single-vector DNA replicon system that permits simultaneous efficient high-level accumulation of two recombinant proteins in the same plant cells (tobacco leaves), allowing the production of 0.5 mg of antibody per gram leaf (fresh weight) within four days from vector infiltration. Mapp Biopharmaceutical Inc., using similar methodology, transiently expressed the humanized antibodies, MB-003 and ZMab, in tobacco leaves. MB-003 and ZMab were later combined and designated as ZMapp. The use of these antibodies as a pharmaceutical drug was found to efficiently cured 100% of Ebola infected primates (rhesus macaque).7
On the other hands, in a recent years,8 Macuna pruriens (L.) (Fabaceae), in which the presence of a high amount of L-DOPA in seeds has been proved, attracted global attention for the possibility to produce this catecholamine precursor from natural resources. In fact, it has been reported that the dried endocarp powder obtained from M. pruriens bean may give some benefit in the treatment of Parkinson’s disease.9
More recently, another big challenge in term of plant-derived drugs utilization is a possible prevention of type-1 diabetes (T1D).10 T1D is a metabolic disease that leads the autoimmune destruction of insulin-producing pancreatic beta cells. T1D is characterized by the presence of several autoantibodies in the serum of patients that are used as markers of beta cell autoimmunity. They include Islet Cell Antibodies (ICA, against cytoplasmic proteins in the beta cell), Glutamic Acid Decarboxylase (GAD-65) antibodies, Insulin Autoantibodies (IAA), and IA (protein tyrosine phosphatase) antibodies.11
It has been proposed that, like a vaccine, oral administration of insulin may induce systemic immunological tolerance and thus prevent or delay the onset of T1D.12 Bertini et al.10 combined edible plant systems, namely spinach (Spinacia oleracea cultivar Industra) and red beet (Beta vulgaris cultivar Moulin Rouge), with the magnICON® expression system to develop a safe, cost-effective and environmentally sustainable platform for the large-scale production of GAD65. It is worth mentioning that the GAD65 autoantibodies are also involved in several neurological diseases such as in some of epileptic patients, Stiff Person Syndrome (SPS), cerebellar ataxia, and autoimmune encephalitis13 opening possible future application of such biotechnological platform.
In conclusion, plant-based protein/drug production for industrial and clinical applications, offers a promising technology that could be complementary or alternative to the conventional bio-production systems.
However, some burden in apply this useful technologies has to been considered, such as the lack of adequate international regulation that guarantee a good manufactural practice in term of genetically modified plants and that control the pharmaceuticals platforms involved in the production of these medicals compounds.
It is desirable that many efforts will be employed in this way in order to guarantee to a majority part of world population the possibility to access these new medical compounds.
None.
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.

©2018 Lombardi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.