MOJ
eISSN: 2471-139X


Research Article Volume 3 Issue 2
Department of Cell Biology & Physiology, University of Salzburg, Austria
Correspondence: Katharina Erlbacher, Division of Animal Structure & Function, Department Cell Biology & Physiology, University of Salzburg, Austria, Tel 4366280445607, Fax 436628044745607
Received: February 02, 2017 | Published: March 6, 2017
Citation: Erlbacher KMT, Minnich B. Long-term hypophagic effect of chronic cannabis (Δ9-thc) administration on body weight progress investigated in a rat model. MOJ Anat Physiol. 2017;3(2):64-69. DOI: 10.15406/mojap.2017.03.00088
Background: The use of cannabis to prevent weight gain or weight loss as unwanted side effects complementary to certain primary therapies inducing such side effects is still under study. Previous studies of different dose- and time-dependent D9-Tetrahydrocannabinol treatment approaches have shown contradictory effects on food intake behavior and body weight changes. Here we examined the effect of a daily dose of 3mg/kg over a period of up to 8.5month in a rat model which is equivalent to about 0.25grams over a period of 21.5years in terms of human life spans. Moreover, this effect was investigated in both, a native start generation and a D9-THC-biased offspring filial generation.
Methods: In this open, controlled study 48 nude rats (f+m) originating from two different generations were separated in D9-THC-treatment- and control groups. The daily administration of THC and control vehicle was done intraperitoneally. Body weight change was recorded at 14(SG) and 9(F1) time points throughout the study.
Results: In addition to a clear hypophagic effect found with long-term THC treatment, we observed an immediate cessation of this effect after settlement of the drug during recurrent lactation periods in both, SG and F1 generations. Thus the mechanisms involved in weight loss after chronic THC treatment at this dose-rate seem to remain reversible over a long period of time.
Conclusion: We conclude that long-term THC administration at a dose level of 3mg/kg might be suitable for complementary therapies effecting controlled weight loss when intended (e.g. treatment of type-2 diabetes, obesity, lack of sufficient sleep (insomnia), some types of cancer, etc.).
Keywords: d9-tetrahydrocannabinol, long-term treatment, hypophagia, complementary therapy, weight control
BW, body weight; F1, filial generation 1; SG, start generation; THC, d9-tetrahydrocannabinol
In the course of a study examining the effects of chronic ∆9-THC administration on the gonadal vascularization and on the protective potential of the cornified envelope of the epidermis in nude rats (unpublished data) throughout different hereditary-biased filial generations we discovered an unexpected reversible hypophagic effect of THC caused by regular treatment intermissions during lactation and nursing periods of the native start generation (SG) and filial generation one (F1).
The potential of D9-Tetrahydrocannabinol (THC) to serve either to stimulate or retard appetite has still not been explored in full detail. Diverse findings of previous studies show contradictory effects of THC on body weight. Basically, dose-dependent and time-dependent differences have been reported. For therapeutic use it is of great interest as to how THC should be administered in order to obtain an optimal beneficial effect depending on the objective of treatment. Such objectives are on the one hand unwanted weight gain caused by appetite enhancement due to various first line therapies in the treatment of type-2 diabetes, lack of sufficient sleep (insomnia, stress) etc. in turn resulting in reduced levels of the metabolism regulating hormones leptin and ghrelin as well as simple physical inactivity. On the other hand it is objected to treat patients suffering from severe appetite loss or muscle wasting (e.g. in cancer, cystic fibrosis, AIDS, anorexia etc.).
D9-Tetrahydrocannabinol (THC) is shown to have the ability, as partial agonist, to activate cannabinoid type-1 (CB1) and type-2 (CB2) receptors.1‒5 G-protein coupled cannabinoid CB1 receptors which are activated by the endocannabinoid neurotransmitters 2-arachidonoylglycerol and anandamide are located primarily in central and peripheral neurons, whereas CB2 receptors are predominantly in immune cells. CB1 receptors are also expressed by some non-neural cells in various tissues outside the brain (e.g. intestinal tract).6‒11 Thus, in addition to its psychoactive potential D9-THC also influences the ingestive behavior in animals and men. There is clinical evidence for the efficacy of inverse agonists of CB1 receptors (e.g. Rimonabant) for the improvement of the metabolic status in patients suffering from type-2 diabetes and dyslipidaemia but there are adverse psychiatric effects associated with this compound which caused its withdrawal from the market.12
D9-Tetrahydrocannabivarine (THCV), a naturally occurring analogue of THC, behaves as a neutral CB1 antagonist and was found to produce hypophagic effects.13 The activation of CB2 receptor is suggested to improve glucose tolerance after a glucose load14 although other studies with CB2 knockout mice15,16 showed contrary results. Thus the exact role of CB2 receptors still remains unclarified. Bellocchio et al.17 reported a bimodal mode of action of endo-and exogenous cannabinoids in the control of stimulated food intake. According to the neural populations in which CB1 receptors are expressed, their pharmacology may vary. It is suggested that the orexigenic effect of a low dose of THC is mediated by CB1-dependent inhibition of glutamate release, whereas the hypophagic effect of higher doses of THC occurs via CB1-mediated inhibition of GABA release.
Williams et al.18 presented the first comprehensive dose-response and time-course analysis of exogenous cannabinoid effects on eating behavior in rats. With oral application of THC with doses in the range from 0.063 to 2mg/kg they obtained the most marked hyperphagic effect with a 1mg/kg dose. Although these authors found a dose-dependent hyperphagic effect, the study also clearly showed that this effect was an acute response to THC administration which occurred only within 1-2hours after treatment and which was abrogated after 4 hours. Similar dose responses to increased food intake have been presented by Glick & Milloy19 whereby in their study a dose of 2mg/kg decreased food intake. Appetite stimulation after single doses of THC has been found to be highly variable.20 Some studies report a potential long-term benefit of THC on increased appetite in healthy individuals, cancer- and AIDS patients21‒23 even up to 42days. Mattes et al.24 found a significantly elevated mean daily energy intake following chronic dosing (2.5mg/kg) by rectal suppository relative to acute dosing.
In the present study we focus on the long-term effect of medium dosage (3mg/kg) synthetic D9-THC (Dronabinol, 1mg/100µl) on body weight and its reversibility in a rat model. Contrary to most previous studies we note a hypophagic effect induced by chronic THC (i.p. daily) administration throughout a period of 284days. Comparing the life spans of rats and humans THC treatment of 8.5months in rats equals a human drug consumption period of about 21.5years.25 Recurrent settlement of THC administration during nursing periods in female rats clearly shows an immediate reversal of this effect.
Animals and study design
CR (Charles River) hairless mutation rat (Crl: CD-Prss8) of the start generation (SG) were subdivided into two groups:
In periods of twomonths rats from the same group were kept pair wise and were allowed to mate. During the following lactation- and nursing periods THC administration was interrupted. Rats were housed in an air-conditioned specific pathogen free environment with the temperature set at 20˚C-24˚C, 45-65% rH and a 12-hour-light: 12hour dark cycle (200lx). Due to the insufficient thermoregulation of nude rats high energy food (ssniff, Spezialdiäten GmbH, Germany) and water were provided ad libitum.
Assessment of body weight
Body weight (bw) was assessed using an electronic balance at 14(SG) or 9(F1) time points according to study events such as treatment onset, mating, nursing and scarification.
Statistics
Comparisons of body weight data between groups at the different time points were performed using two-tailed Student’s t-tests with a power of 0.8 within 0.05 or 0.01 confidence intervals since all data were normally distributed.
Generation SG
In the start generation (SG) significant differences (p<0.05) in body weight (bw) between controls and THC treated animals were found in male rats only, first occurring after 1 month of THC treatment. These differences in bw steadily increased until 8.5 months of treatment when animals were sacrificed (Table 1 & Figure 1).
Treatment |
Bw 0.25 |
Bw 2.0 |
Bw 2.5 |
Bw 3.0 |
Bw 4.0 |
Bw 5.0 |
Bw 6.0 |
Months |
Months |
Months |
Months |
Months |
Months |
Months |
|
C means |
8g |
350g |
371g |
469g |
523g |
468g |
546g |
C StDev |
1g |
6g |
13g |
7g |
16g |
28g |
20g |
THC means |
9g |
322g |
328g |
410g |
455g |
464g |
478g |
THC StDev |
1g |
41g |
42g |
21g |
18g |
11g |
7g |
Sign. Diff. p |
0.288 n.s. |
0.299 n.s. |
0.166 n.s. |
0.011* |
0.008** |
0.018* |
0.001** |
Treatment |
Bw 6.5 |
Bw 7.0 |
Bw 8.5 |
Bw 9.0 |
Bw 10.0 |
Bw 11.0 |
Bw 11.5 |
Months |
Months |
Months |
Months |
Months |
Months |
Months |
|
C means |
596g |
594g |
613g |
630g |
631g |
638g |
642g |
C StDev |
34g |
37g |
37g |
32g |
43g |
26g |
35g |
THC means |
503g |
510g |
532g |
538g |
544g |
555g |
547g |
THC StDev |
3g |
0g |
7g |
13g |
13g |
16g |
12g |
Sign. Diff. p |
0.009** |
0.017* |
0.021* |
0.010** |
0.029* |
0.009** |
0.011* |
Table 1 Body weight Start Generation: Controls vs THC male rats.
Bw SG: C vs THC male rats (t-test two-tailed, CI: = 0.05, power: = 0.8, n=6/group). THC treatment (3 mg/kg bw i.p.) daily injection started at the age of 2 months. Data of body weight (bw) rounded; C: Control Group; THC: Treatment Group; n.s: Not Significant; * : Significant (p ≤ 0.05); **: Highly Significant (p ≤ 0.01)
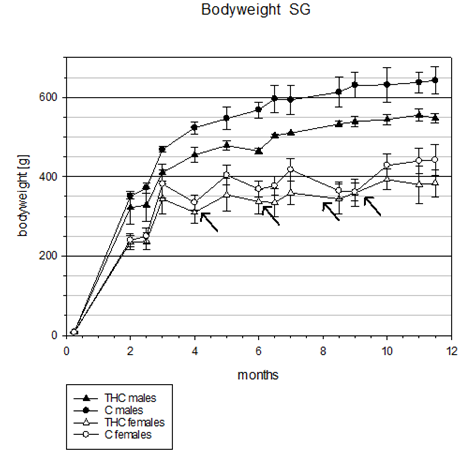
Figure 1 Start generation (SG): Comparison of body weight progress across 11.5 months between chronic THC treated (3 mg/kg bw i.p. daily injection) and control animals. Arrows indicate time points of female lactation periods (1 month after giving birth without treatment) where differences of body weights between THC and control group were decreased. The difference in body weight progress of male animal groups (THC vs. C) increased steadily from the beginning until the end of the study.
No significant differences in bw occurred in female rats, probably due to four THC treatment interruptions (after 3, 5, 7, and 9 months) during late pregnancy and nursing. Even though there was no statistical significance in THC effects on reduced bw after 1 month of treatment comparable to those observed in male rats, a strong tendency was observed (Table 2 & Figure 1). Since the difference in bw after THC treatment interruptions with an average length of 31 days during lactation without THC administration decreased every time between treatment groups (Figure 1, arrows) and increased again after reinstatement of THC treatment it may be suggested that the hypophagic effect of chronic THC administration on bw (reduction) is a reversible process.
Treatment |
Bw 0.25 |
Bw 2.0 |
Bw 2.5 |
Bw 3.0 |
Bw 4.0 |
Bw 5.0 |
Bw 6.0 |
Months |
Months |
Months |
Months |
Months |
Months |
Months |
|
C means |
8g |
240g |
250g |
382g |
335g |
404g |
369g |
C StDev |
1g |
16g |
20g |
35g |
19g |
24g |
21g |
THC means |
8g |
235g |
237g |
344g |
310g |
354g |
337g |
THC StDev |
0.5g |
17g |
21g |
37g |
27g |
42g |
30g |
Sign. Diff. p |
0.576 n.s. |
0.701 n.s. |
0.441 n.s. |
0.226 n.s. |
0.203 n.s. |
0.153 n.s. |
0.206 n.s. |
Treatment |
Bw 6.5 |
Bw 7.0 |
Bw 8.5 |
Bw 9.0 |
Bw 10.0 |
Bw 11.0 |
Bw 11.5 |
Months |
Months |
Months |
Months |
Months |
Months |
Months |
|
C means |
377g |
418g |
365g |
361g |
428g |
440g |
442g |
C StDev |
24g |
28g |
22g |
22g |
28g |
32g |
39g |
THC means |
335g |
359g |
344g |
359g |
393g |
380g |
383g |
THC StDev |
35g |
30g |
38g |
34g |
26g |
47g |
34g |
Sign. Diff. p |
0.165 n.s. |
0.071 n.s. |
0.462 n.s. |
0.936 n.s. |
0.183 n.s. |
0.147 n.s. |
0.122 n.s. |
Table 2 Body weight Start Generation: Controls vs THC male rats.
Bw SG: C vs THC female rats: t-test two-tailed, CI: = 0.05, power: = 0.8, n=6/group: THC treatment (3 mg/kg bw i.p.) daily injection started at the age of 2 months. Females were giving birth after 3, 5, 7, and 9 months (weight loss). Data of body weight (bw) rounded; C: Control Group; THC: Treatment Group; n.s: Not Significant; *: Significant (p ≤ 0.05); **: Highly Significant (p ≤ 0.01)
Generation F1
Similar effects of chronic THC treatment resulting in highly significant lower body weights after 3 months of treatment (p <0.01) in comparison to control animals were observed in filial generation 1-male animals (Table 3 & Figure 2).
Treatment |
Bw 1.0 |
Bw 1.5 |
Bw 2.0 |
Bw 3.5 |
Bw 4.0 |
Months |
Months |
Months |
Months |
Months |
|
C means |
110g |
217g |
348g |
474g |
489g |
C StDev |
1.5g |
2g |
3g |
7g |
14g |
THC means |
109g |
207g |
329g |
425g |
447g |
THC StDev |
4g |
6g |
12g |
9g |
14g |
Sign. Diff. p |
0.768 n.s. |
0.049* |
0.054 n.s. |
0.001** |
0.022* |
Treatment |
Bw 5.0 |
Bw 6.0 |
Bw 6.5 |
Bw 7.5 |
Months |
Months |
Months |
Months |
|
C means |
527g |
554g |
565g |
595g |
C StDev |
11g |
7g |
11g |
16g |
THC means |
465g |
491g |
502g |
505g |
THC StDev |
10g |
15g |
16g |
18g |
Sign. Diff. p |
0.001** |
0.002** |
0.0001** |
0.003** |
Table 3 Body weight Filial Generation 1: Controls vs THC male rats.
Bw F1: C vs THC male rats (t-test two-tailed, CI: a = 0.05, power: b = 0.8, n=6/group). THC treatment (3 mg/kg bw i.p.) daily injection started at the age of 1 month. Data of body weight (bw) rounded; C: control group; THC: Treatment Group; n.s: Not Significant; *: Significant (p ≤ 0.05); **: Highly Significant (p ≤ 0.01).
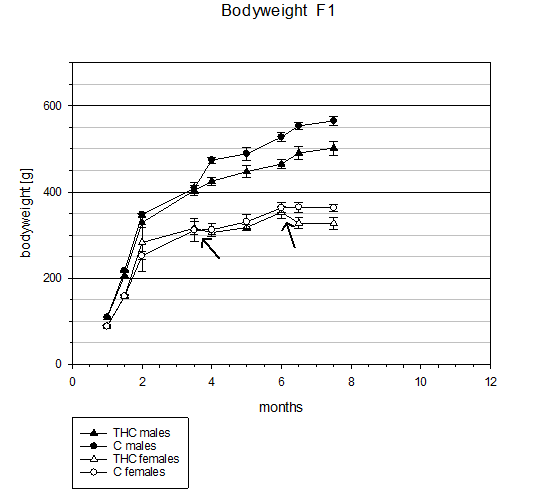
Figure 2 Filial generation 1 (F1): Comparison of body weight progress across 7.5 months between chronic THC treated (3 mg/kg bw i.p. daily injection) and control animals. Arrows indicate time points of female lactation periods (1 month after giving birth without treatment) where no differences of body weights between THC and control group were found. After 3 months of THC treatment the difference in body weight progress of male animal groups increased steadily until the end of the study.
Moreover, in female animals there were also significant (p<0.05) differences in body weights which developed about 1 month after giving birth for the second time (i.e. months 6 and 6.5) (Table 4 & Figure 2). The difference in bw after THC treatment interruptions with an average length of 43 days during lactation without treatment decreased every time between treatment groups (Figure 2, arrows) and increased again after reinstatement of THC treatment also in this generation. This again indicates that the hypophagic effect of chronic THC administration on bw (reduction) using a dosage of 3 mg/kg is a reversible process.
Treatment |
Bw 1.0 |
Bw 1.5 |
Bw 2.0 |
Bw 3.5 |
Bw 4.0 |
Months |
Months |
Months |
Months |
Months |
|
C means |
88g |
158g |
252g |
312g |
331g |
C StDev |
5g |
2.5g |
9g |
16g |
17g |
THC means |
89g |
159g |
283g |
306g |
318g |
THC StDev |
1.5g |
4g |
67g |
9g |
7g |
Sign. Diff. p |
0.754 n.s. |
0.909 n.s. |
0.475 n.s. |
0.554 n.s. |
0.280 n.s. |
Treatment |
Bw 5.0 |
Bw 6.0 |
Bw 6.5 |
Bw 7.5 |
Months |
Months |
Months |
Months |
C means |
363g |
365g |
363g |
376g |
C StDev |
12g |
12g |
8g |
8g |
THC means |
353g |
328g |
327g |
358g |
THC StDev |
14g |
12g |
14g |
18g |
Sign. Diff. p |
0.395 n.s. |
0.019* |
0.017* |
0.198 n.s. |
Table 4 Body weight Filial Generation 1: Controls vs THC Female rats.
Bw F1: C vs THC Female rats (t-test two-tailed, CI: a = 0.05, power: b = 0.8, n=6/group). THC treatment (3 mg/kg bw i.p.) daily injection started at the age of 1 month. Data of body weight (bw) rounded; C: control group; THC: Treatment Group; n.s: Not Significant; *: Significant (p ≤ 0.05); **: Highly Significant (p ≤ 0.01)
Since CB1 and CB2 receptors are coupled to G-proteins and the activation of these receptors by D9-THC induces a decoupling of G-proteins, in turn causing intracellular inhibition of the Adenylylcyclase enzyme and following second messenger cascades, this tremendously influences cell metabolism. Different dose- and time-dependent THC treatment approaches did show contradictory effects on food intake behavior and body weight changes. In our study we could clearly show that a dose of 3mg/kg over a long period resulted in markedly reduced body weight progress and thus might assist therapeutic strategies to decrease body weight in cases where intended. This observed hypophagic effect probably is due to either glucose tolerance- or drug absorption effects since there was no aversive food intake- or psychogenic behavior observed in the THC group. While short term administration of low doses (≤1mg/kg) THC usually increases food intake,18 long term administration of a dose of 2mg/kg leads to weight loss19 which is in agreement with our findings. Recent publications of Le Foll et al.26 and Sansone & Sansone27 also proposed THC chronic treatment to produce weight loss, is associated with lower body mass index, and maybe a useful therapeutic for the treatment of obesity and its complications. Our results are in contrast to Abel28 who concluded that after an initial period of weight loss, animals given cannabis begin to increase in body weight at a rate similar to that of control animals. Other long-term studies21‒24 have also found hyperphagic effects of chronic THC treatment which we cannot confirm here.
Moreover, we observed an immediate cessation of the long-term hypophagic effect after settlement of the drug during recurrent lactation periods in both SG and the F1 generation. Thus mechanisms involved in weight loss after chronic THC treatment at this dose-rate seem to remain reversible over a long period of time. Consumption of 3mg/kg per day (equals approx. 0.25 grams in humans) reflects dosages typical of light marijuana smokers whereas heavy smokers on average smoke a gram and a half to two grams of concentrate per day. The effect of such high doses on body weight change still remains unknown. In addition, different ways of drug administration (oral, rectal, smoke, intraperitoneal) may also cause divergent metabolic effects or food intake behavior and thus need to be explored in further detail.
We suggest that since no long lasting hypophagic effects of chronic THC treatment after settlement are observed, dose dependent chronic THC administration is suitable for complementary therapies effecting controlled weight loss when intended (e.g. complementary to various first line therapies in the treatment of type-2 diabetes, obesity, lack of sufficient sleep (insomnia), stress syndromes, some types of cancer, etc.).
The authors acknowledge Ms. Christine Radner, BSc. for her excellent laboratory assistance.
Author declares that there is no conflict of interest.

©2017 Erlbacher, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.