MOJ
eISSN: 2471-139X


Research Article Volume 6 Issue 6
PG and Research Department of Zoology, Government Arts College (Autonomous), India
Correspondence: Veeramani A, PG and Research Department of Zoology, Government Arts College (Autonomous), Kumbakonam, 612 002, Tamil Nadu, India, Tel 8124881520
Received: August 24, 2019 | Published: December 5, 2019
Citation: Veeramani A, Ramkumar R, Keerthika M. Impact on feeding and growth rate of millipedes and their conservation needs in Cauvery Delta Region of Kumbakonam, Tamil Nadu. MOJ Anat & Physiol. 2019;6(6):205-212. DOI: 10.15406/mojap.2019.06.00274
Diplopoda (millipedes) is one of the largest classes of animal kingdom belonging to the Devonian period constituting the third largest group after Insecta and Arachnida in terrestrial arthropods. These diplopods play an ample role in soil formation being involved both physical and chemical factors. In addition, millipedes can be as a bio-indicator for environmental changes in ecosystems. A study on millipedes such as Yellow-spotted (Harpaphe haydeniana), Red-spined (Xenobolus carnifes), Colossal slender spined (Spinotarsus colosseus) and American giant, (Narceus americanus) were conducted along the bank of Cauvery River in Kumbakonam region. The present study is intended to find out to know the impact on food intake and the growth rate of millipedes by artificial feeding of decomposed dry leaves and the amount of milli casts (faecal) produced of millipedes under captive condition.
The experiment was carried out during the monsoon season and hand picking of millipedes were done. Ten numbers each of four species of millipedes were kept in separate plastic jars. Dry and decayed leaves were weighed and kept inside the plastic jar as food for millipedes. Before starting the experiment all the individuals of the millipedes were weighed separately and inserted in to the jars. The milli cast deposited by millipedes were separated and weighed to know the production of amount of milli casts. In the case of Harpaphe haydeniana the increase of body weight of 10 individuals shows that there is significant differences between the individuals (F=0.1308, df=8.141, p=0.9969). Xenobolus carnifex shows that there is about 25% of the weight increase among the individuals (F=0.1385, df=8.134, p=0.9962). Spinotarsus colosseus the increase of body weight of 10 individuals shows that there is a significant differences between the individuals (F=0.05101, df=8.142, p=0.9999). Narceus americanus the 90 days period of experiment shows that there is about 25% of the weight increase among the individuals (F=0.1308, df=8.141, p=0.9969). It is suggested that the results of this experiment can be applied to millipedes in their natural setting, further confirming millipedes as important components of soil ecosystems and nutrient cycling.
Keywords: millipedes, body weight, food intake, milli cast, conservation, Cauvery
The saprophagous macrofauna (eg. millipedes, earthworms, slugs) are mainly responsible for increased nutrient leaching from dead plant organic matter and enhancement of soil humification.1,2 Diplopoda (millipedes) is one of the largest classes of animal kingdom belonging to the Devonian period constituting the third largest group after Insecta and Arachnida in terrestrial arthropods.3,4 Based on the body structure, five morphotypes have been identified in millipedes by Kime & Golovatch5. Polyxemda (bristly millipedes or bark-dwellers), Glomenda (pill millipedes or rollers), Julida (bulldozers or rammers), Polydesmida (wedge or litter-splitters) and Platydesmida (borers). Millipedes occupy three major habitats in forest ecosystem (the aerial part of vegetation, bark and rotten woody stumps, litter and soil strata).6
Similarly, they also exhibit horizontal distribution depending on the diversity and quantity of plant litter on the forest floor or soil. Unlike earthworms and woodhce, millipedes are conservative and very sensitive to water deficit and fail to overcome the limitation of even a single edaphic factor (eg soil texture, litter thickness) although rest of the ecological conditions are conducive.5 Millipedes are more abundant in Western Ghat forests than in grasslands or coastal habits due to relative abundance of litter biomass.7,8
These diplopods play an ample role in soil formation being involved both physical and chemical factors. Physical disruption of the upper layer of the soil and the litter noticeably enhances the burrowing activities of both adult and larval stadia. Many immature stadial durations are completed in the humus layer of the soil itself. Chemical influences of these animals on soil are of several kinds (1) modification of plant material through digestion (2) uptake and concentration of calcium and other minerals and (3) release of nitrogenous compounds from metabolic excretion and formation of weak organic acids as the result of death and protein breakdown.9 South Indian millipedes are known to feed on soil and litter in varying stages of decomposition. Most of the tropical millipedes are known to aestivate during drought, their distribution and period of activity are dependent on humidity and water resources of the soil. Many of these also feed on green vegetable matter and in the absence of the litter they switch over to utilization of soil, rich in rotting organic matter.
Millipedes are functionally important in facilitating nutrient cycling and decomposition of dead plant tissues. In addition, millipedes can be a bioindicators for environmental changes in ecosystems. There is no study on millipedes were reported in the Cauvery delta region, even though we have many species of millipedes present here. There is a study on diversity of millipedes were reported in Alagar Hill Reserved Forest, Madurai by Alagesan & Ramanathan10. Apart from this studies are meager in Tamil Nadu particularly in Cauvery delta region. Hence a study on millipedes such as Yellow-spotted (Harpaphe haydeniana), Red-spined (Xenobolus carnifes), Colossal slender spined (Spinotarsus colosseus) and American giant, (Narceus americanus) were conducted along the bank of Cauvery River in Kumbakonam region with the objectives of the growth rate of four different species of millipedes under captive condition, food intake of millipedes by artificial feeding of decomposed dry leaves and the amount of milli casts (faecal) produced by four different millipede species.
Kumbakonam is located at 10.97°N 79.42°E. It lies in the region called the "Old delta" which comprises the north-western taluks of Thanjavur district that have been naturally irrigated by the waters of the Cauvery and its tributaries for centuries in contrast to the "New Delta" comprising the southern taluks that were brought under irrigation by the construction of the Grand Anicut canal and the Vadavar canal in 1934. It has an average elevation of 26 metres. The town is bounded by two rivers, the Cauvery River on the north and Arasalar River on the south (Figure 1).
The experiment was carried out during the north-west monsoon (December–January) and post monsoon (February and March) when millipedes are available in large numbers. Hand picking of millipedes were carried out to conduct the experiment. The experiment consisted of 10 numbers each of four species of millipedes were kept in separate plastic jars. Dry and decayed leaves were weighed and kept inside the plastic jar as food for millipede. Although spraying of water are also done to keep the cool environment. The experiment helps to assess no-choice of feeding of captive millipedes under laboratory condition. After the initiation period, the millipedes were allowed to feed the diets.
Set-up of the experiments
Plastic jars with well-fitting perforated lids were used for this experiment. Jars were large enough to contain the amount of air needed for the millipede to breathe normally during a 24-h period. Before starting the experiment all the individuals of the millipedes were weighed separately and inserted in to the jars. Regular weight of the millipedes was taken to know the growth. Similarly same quantity of dry and decayed leaves were weighed and kept inside the jars and water was sprayed for make wet of the leaves. Feed was given whenever the leaves were fed by millipedes. Care was taken to provide same quantity of leaves at every time. Every 15 days of interval the unfed leaves were weighed and removed. The faeces (milli cast) deposited by millipedes were separated and weighed to know the production of amount of milli casts. The same procedure was continued till the end of the experiment. Digital weighing balance was used to estimate the accurate weight of millipedes and feed.
Based on these experiment, data were collected on amount of food intake and milli cast produced by millipedes. Also the increasing trend of body weight of millipedes was also collected. Care was taken to remove and weighing of the millipedes, because of some imperfections such as defensive body secretions on the tweezers and water condensation on the tweezers’ fingers may have occurred. The average difference between the calculated weight of all the species of millipedes was used to correct the directly measured weight gain.
In order to calculate the consumption index (CI) the formula of Waldbauer11 was slightly modified:
(1)
The efficiency of conversion of ingested food (ECI; %) into body substance of the millipedes of each jar was calculated using Equation 2:11,12
(2)
Initial body weight per 10 millipedes (at t=0), their weight after 15 days (at t=15), food intake, body weight gain, and CI and ECI were calculated. The computer programme PAST and MINITABT was used for the statistical analyses.
Growth rate
The results collected for 90 days from mid December 2019 to mid March 2019 on four species of millipedes shows that there is a considerable increase of growth from the initial and final weight of the experiment. In the case of Harpaphe haydeniana the increase of body weight of 10 individuals shows that there is significant differences between the individuals (F=0.1308, df=8.141, p=0.9969). Similarly more than 50% of the body weight increase were seen some of the individuals (Table 1).
Harpaphe haydeniana |
|
|
||||||||
Individuals |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Initial weight (g) |
0.58 |
0.64 |
0.34 |
0.39 |
0.39 |
0.39 |
0.47 |
0.37 |
0.45 |
0.42 |
Final weight (g) |
0.85 |
0.85 |
0.75 |
0.74 |
0.63 |
0.55 |
0.66 |
0.77 |
0.65 |
0.67 |
Difference weight (g) |
0.27 |
0.21 |
0.41 |
0.35 |
0.24 |
0.16 |
0.19 |
0.4 |
0.2 |
0.25 |
Table 1 Growth rate of Harpaphe haydeniana for the period of 90 days
The graph showing that the difference between initial and final experiment of ninety days period of 10 individuals shows that there is a peak increase of weight in the individuals 3 and 8 because of the tremendous growth of the individuals in the final stage of the experiment (Figure 2).
The growth rate of 10 individuals of Harpaphe haydeniana at every 15 days interval shows that there is a steady increase of the body weight of each individual (Figure 3). The 15 days interval of experiment the growth was high in the individuals 3 and 8.
In the case of Xenobolus carnifex the 90 days period of experiment shows that there is about 25% of the weight increase among the individuals (Table 2). There is a significant differences in growth between the individuals (F=0.1385, df=8.134, p=0.9962).
Xenobolus carnifex |
|
|
|
|
|
|
|
|
|
|
Individuals |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Initial Weight (g) |
0.93 |
0.86 |
0.82 |
0.78 |
0.72 |
0.8 |
0.98 |
1.06 |
1.36 |
1.35 |
Final Weight (g) |
1.18 |
1.09 |
1.07 |
1.03 |
0.96 |
1.05 |
1.24 |
1.38 |
1.69 |
1.54 |
Difference between Initial and Final Weight (g) |
0.25 |
0.23 |
0.25 |
0.25 |
0.24 |
0.25 |
0.26 |
0.32 |
0.33 |
0.19 |
Table 2 Growth rate of Xenobolus carnifex for the period of 90 days
The graph shows that there is a s<li class="ref" id="ref1"> increasing trend was seen in all the stages (initial, final and difference) between the individuals (Figure 4). Similarly the individuals between 15 days interval also shows that there is straight increase of growth (Figure 5).
In the case of Spinotarsus colosseus the increase of body weight of 10 individuals shows that there is a significant differences between the individuals (F=0.05101, df=8.142, p=0.9999). Similarly more than 50% of the body weight increases were seen in some of the individuals (Table 3).
Spinotarsus colosseus |
|
|
|
|
|
|
|
|
|
|
Individuals |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Initial weight (g) |
0.58 |
0.58 |
0.55 |
0.65 |
0.47 |
0.56 |
0.64 |
0.64 |
0.55 |
0.72 |
Final weight (g) |
0.69 |
0.78 |
0.69 |
0.74 |
0.6 |
0.66 |
0.77 |
0.77 |
0.71 |
0.88 |
Difference between initial and final weight (g) |
0.11 |
0.2 |
0.14 |
0.09 |
0.13 |
0.1 |
0.13 |
0.13 |
0.16 |
0.16 |
Table 3 Growth rate of Spinotarsus colosseus for the period of 90 days
The graph showing that the difference between initial and final experiment of ninety days period of 10 individuals shows that there is a peak increase of weight in the individuals 3, 5 and 9 because of the tremendous growth of the individuals in the final stage of the experiment (Figure 6).
The growth rate of 10 individuals of Spinotarsus colosseus at every 15 days interval shows that there is a steady increase of the body weight of each individual (Figure 7). The 15 days interval of experiment, the growth was high in the individuals 2, 9 and 10.
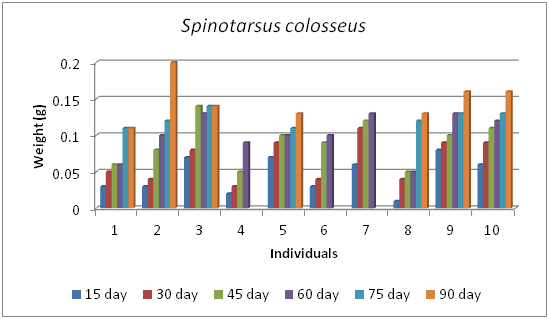
Figure 7Trend of growth rate between individuals at every 15 days interval of Spinotarsus colosseus.
In the case of Narceus americanus the 90 days period of experiment shows that there is about 25% of the weight increase among the individuals (Table 4). There is a significant differences between the individuals (F=0.1308, df=8.141, p=0.9969).
Narceus americanus |
||||||||||
Individuals |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Initial weight (g) |
7.34 |
5.34 |
8.31 |
5.68 |
4.86 |
5.58 |
5.36 |
5.91 |
6.12 |
5.05 |
Final weight (g) |
8.31 |
6.17 |
8.78 |
5.99 |
5.59 |
6.54 |
6.03 |
6.23 |
7.14 |
5.6 |
Difference between initial and final weight (g) |
0.97 |
0.83 |
0.47 |
0.31 |
0.73 |
0.96 |
0.67 |
0.32 |
1.02 |
0.55 |
Table 4 Growth rate of Narceus americanus for the period of 90 days
The graph shows that there is a s<li class="ref" id="ref1"> increasing trend was seen in all the stages (initial, final and difference) between the individuals (Figure 8). Similarly the individuals between 15 days interval also shows that there is straight increase of growth (Figure 9).
Food intake and faecal production
The dry and decayed leaves were provided as food for all the individuals of the millipedes shows that there is positive and negative trends were seen among the individuals (Table 5). The statistical analysis of the food intake and faecal production shows that there is a significant trends (F=0.3206, df=8.121, p=0.9453).
Harpaphe haydeniana |
||||||||||
Individuals |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Total food intake (g) |
0.88 |
2.34 |
3.64 |
5.19 |
4.8 |
5.4 |
4.06 |
4.51 |
4.07 |
6.59 |
Total faecal production (g) |
3.37 |
4.15 |
3.21 |
5.15 |
4.54 |
5.52 |
5.51 |
5.02 |
3.57 |
2.98 |
Difference between food intake and faecal production (g) |
-2.49 |
-1.81 |
0.43 |
0.04 |
0.26 |
-0.12 |
-1.45 |
-0.51 |
0.5 |
3.61 |
Table 5 Differences between food intake and faecal production of Harpaphe haydeniana
The graph shows that there are positive and negative trends in food intake and faecal production (Figure 10). Figure 11 shows that the total food intake and total growth of 90 days period of Harpaphe haydeniana.
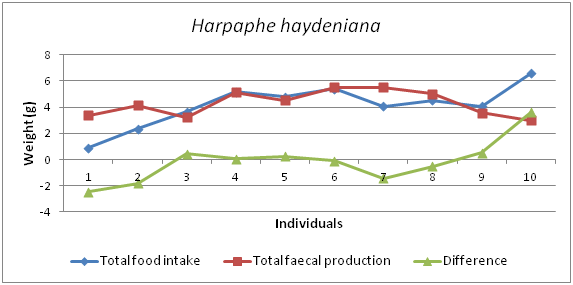
Figure 10 Graph showing the differences between Food intake and faecal production of Harpaphe haydeniana.
The food provided for all the individuals of the Xenobolus carnifex millipedes shows that there is positive trends were seen among the individuals (Table 6). The statistical analysis of the food intake and faecal production shows that significant trends (F=0.3092, df=8.142, p=0.9505).
Xenobolus carnifex |
||||||||||
Individuals |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Total food intake (g) |
4.45 |
4.77 |
5.23 |
4.83 |
6.68 |
4.37 |
2.93 |
6.15 |
5.23 |
3.77 |
Total faecal production (g) |
2.54 |
2.98 |
1.84 |
2.69 |
4.28 |
3.14 |
2.77 |
2.71 |
3.61 |
2.88 |
Difference between food intake and faecal production (g) |
1.91 |
1.79 |
3.39 |
2.14 |
2.4 |
1.23 |
0.16 |
3.44 |
1.62 |
0.89 |
Table 6 Differences between food intake and faecal production of Xenobolus carnifex
The graph shows that there are positive trends in food intake and faecal production (Figure 12). Figure 13 shows that s<li class="ref" id="ref1"> trend of the total food intake and total growth of 90 days period of Xenobolus carnifex.

Figure 12Graph showing the differences between Food intake and faecal production of Xenobolus carnifex.
The dry and decayed leaves were provided as food for all the individuals of the Spinotarsus colosseus millipedes shows that there is positive and negative trends were seen among the individuals (Table 7). The statistical analysis of the food intake and faecal production shows that significant trends (F=0.5196, df=8.119, p=0.8259).
Spinotarsus colosseus |
|
|||||||||
Individuals |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Total food intake (g) |
7.13 |
9.59 |
4.63 |
5.8 |
5.73 |
7.18 |
7.29 |
7.92 |
5.36 |
10.43 |
Total faecal production (g) |
1.36 |
4 |
1.78 |
2.33 |
3.13 |
1.38 |
3.41 |
3.33 |
2.03 |
4.6 |
Difference between food intake and faecal production (g) |
5.77 |
5.59 |
2.85 |
3.47 |
2.6 |
5.8 |
3.88 |
4.59 |
3.33 |
5.83 |
Table 7 Differences between food intake and faecal production of Spinotarsus colosseus
The graph shows that there are positive and negative trends in food intake and faecal production (Figure 14). Figure 15 shows that the total food intake and total growth of 90 days period of Spinotarsus colosseus.
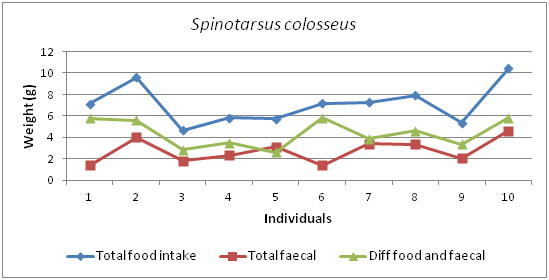
Figure 14 Graph showing the differences between Food intake and faecal production of Spinotarsus colosseus.
The food provided for all the individuals of the Narceus americanus millipedes shows that there is positive trends of increasing food intake were seen among the individuals (Table 8). The statistical analysis of the food intake and faecal production shows that significant trends (F=0.1308, df=8.141, p=0.9969).
Narceus americanus |
||||||||||
Individuals |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
Total food intake (g) |
25.52 |
20.56 |
26.65 |
19.98 |
18.51 |
25.95 |
27.17 |
24.88 |
24.08 |
21.37 |
Total faecal production (g) |
13.33 |
13.48 |
15.82 |
12.97 |
14.25 |
14.61 |
14.83 |
14.06 |
14.24 |
13.11 |
Difference between food intake and faecal production (g) |
12.19 |
7.08 |
10.83 |
7.01 |
4.26 |
11.34 |
12.34 |
10.82 |
9.84 |
8.26 |
Table 8 Differences between food intake and faecal production of Narceus americanus
The graph shows that there are positive trends in food intake and faecal production (Figure 16). Figure 17 shows that s<li class="ref" id="ref1"> trend of the total food intake and total growth of 90 days period of Xenobolus carnifex.
This study demonstrated that all the four species such as Yellow-spotted (Harpaphe haydeniana), Red-spined (Xenobolus carnifes), Colossal slender spined (Spinotarsus colosseus) and American giant, (Narceus americanus) consumed dry and decayed litters. These laboratory results support the results of a field study that showed greater abundance of millipedes in Douglas-fir plots than in western redcedar and western hemlock.13 The presence of leaf litters may be an important determinant of habitat distribution of these species of millipedes may contribute to significant nutrient release in its habitat. It is not clear which aspects of litter quality determine its palatability to millipedes. It is referred from the previous studies that many ericaceous shrubs, such as salal, are known to produce high levels of allelochemicals14 that, if retained in leaf litter, may deter feeding by fauna. However, Neuhauser & Hartenstein15 found no correlation between phenolic content and litter preferences of millipedes or isopods. The food intake and growth rate of these millipedes shows this habitat is suitable for the millipede diversity of various detrivorous animal species.
The higher litter intake per body weight of millipedes may be explained by the lower resource quality of litter compared with the deciduous litter used in most European studies. Extrapolations of laboratory results to field consumption rates provide only conservative approximations, as pointed out by Dangerfield16. Most controlled studies, including ours, may exaggerate levels of coprophagy and underestimate actual field consumption rates. Under laboratory conditions, millipedes have restricted feeding choices and may be forced to consume their own food at greater rates than under natural conditions.16 Furthermore, annual litter fall is likely very conservative, because millipede densities are usually underestimated by field surveys, so our values of 2g/m2 may be on the low side. In addition to millipedes, other invertebrates including earthworms, molluscs, isopods, arthropods, and other micro organisms are expected to feed on litter. For example, in Vancouver Island, another large Xystodesmid millipede (Tubaphe levii Causey) was found on mesic sites17,18 and likely plays a similar role in litter breakdown. We conclude, therefore, that at least a major part of the litter produced in the study sites is probably consumed and thus transformed by soil macrofauna.c
It is concluded that millipedes can impact leaf litter decomposition both directly and indirectly, but the extent of their effect depends on their density and the quality of the substrate (leaf lignin content). Directly, it is clear that millipedes fragment litter, which in some cases has been shown to indirectly increase microbial biomass. It is observed that we could not find an increase but a decrease of soil microbial biomass over time, yet microbial biomass was not affected by the presence of millipedes, suggesting that other factors might have been driving this trend. Millipedes are not the only arthropods influencing decomposition, and their interactions with other organisms in their natural environment could affect the extent of their influence. Notwithstanding, it is suggested that the results of this experiment can be applied to millipedes in their natural setting, further confirming millipedes as important components of soil ecosystems and nutrient cycling. Conservation of these millipedes is very important because there is no doubt they are the friend of farmers.
The authors declare there are no conflicts of interest.

©2019 Veeramani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.